Targeting of (D)MinC/MinD and (D)MinC/DicB complexes to septal rings in Escherichia coli suggests a multistep mechanism for MinC-mediated destruction of nascent FtsZ rings - PubMed (original) (raw)
Targeting of (D)MinC/MinD and (D)MinC/DicB complexes to septal rings in Escherichia coli suggests a multistep mechanism for MinC-mediated destruction of nascent FtsZ rings
Jay E Johnson et al. J Bacteriol. 2002 Jun.
Abstract
The MinC protein is an important determinant of septal ring positioning in Escherichia coli. The N-terminal domain ((Z)MinC) suppresses septal ring formation by interfering with FtsZ polymerization, whereas the C-terminal domain ((D)MinC) is required for dimerization as well as for interaction with the MinD protein. MinD oscillates between the membrane of both cell halves in a MinE-dependent fashion. MinC oscillates along with MinD such that the time-integrated concentration of (Z)MinC at the membrane is minimal, and hence the stability of FtsZ polymers is maximal, at the cell center. MinC is cytoplasmic and fails to block FtsZ assembly in the absence of MinD, indicating that recruitment of MinC by MinD to the membrane enhances (Z)MinC function. Here, we present evidence that the binding of (D)MinC to MinD endows the MinC/MinD complex with a more specific affinity for a septal ring-associated target in vivo. Thus, MinD does not merely attract MinC to the membrane but also aids MinC in specifically binding to, or in close proximity to, the substrate of its (Z)MinC domain. MinC-mediated division inhibition can also be activated in a MinD-independent fashion by the DicB protein of cryptic prophage Kim. DicB shows little homology to MinD, and how it stimulates MinC function has been unclear. Similar to the results obtained with MinD, we find that DicB interacts directly with (D)MinC, that the (D)MinC/DicB complex has a high affinity for some septal ring target(s), and that MinC/DicB interferes with the assembly and/or integrity of FtsZ rings in vivo. The results suggest a multistep mechanism for the activation of MinC-mediated division inhibition by either MinD or DicB and further expand the number of properties that can be ascribed to the Min proteins.
Figures
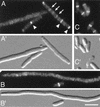
FIG. 1.
Inhibition of Z-ring assembly by MinC/DicB. Fluorescence (A to C) and corresponding DIC (A′ to C′) micrographs showing the distribution of GFP-FtsZ in cells which either were (A and B) or were not (C) subjected to MinC/DicB-mediated division inhibition. Cells of strain PB147(λDR120) [Δ_minDE_(Plac::gfp-ftsZ)] carrying either pJE44 [PBAD::_dicB_] (A and B) or vector pBAD33 (C) were grown at 37°C in the presence of 37 μM IPTG to an OD600 of 0.2. Arabinose was added to 0.05%, and growth was allowed to continue for 60 (A) or 180 (B and C) min. Arrowheads (A), positions of strongly fluorescent rings comparable to those observed in normally dividing cells (C); arrows, positions of some faintly fluorescent structures. Cells were chemically fixed before examination. Both bright and faint rings were included in the data shown in Table 2. Bar, 4 μm.
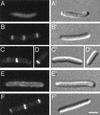
FIG. 2.
Different effects of MinD and DicB on the distribution of GFP-MinC. Micrographs show the distribution of GFP-MinC in the presence of MinD (D) or DicB (B and C) or in the absence of either activator (A). Shown are cells of strain PB114/pLL18 [Δ_minCDE_/_c_I857, PλR::_gfp-minC_], lysogenic for either λDB182 [Plac::_dicB_] (A to C) or λDR155 [Plac::_minD_] (D). Cells were grown at 37°C in the presence of either 0.1% glucose (A) or 100 μM IPTG (B to D) and were chemically fixed prior to examination. Bar, 4 μm.
FIG. 3.
Mutually dependent accumulation of DMinC and DicB on rings. Micrographs show the distribution of GFP-DMinC (A to C) in the absence (A) and presence (B and C) of DicB and that of GFP-DicB (D to F) in the absence (D) and presence (E and F) of DMinC. (A to C) Fixed cells of strain PB114(λDB182)/pLL13 [Δ_minCDE_(Plac::dicB)/c_I857, PλR::gfp-minC(14-231)] which were grown at 37°C in the absence (A) or presence (B and C) of 100 μM IPTG. (D to F) Fixed cells of strain PB114(λJE39)/pJE46 [Δ_minCDE(Plac::gfp-dicB)/_c_I857, PλR::minC(14-231)] which were grown in the presence of 37 μM IPTG at 30°C (D) or 37°C (E and F). Bar, 2 μm.
FIG. 4.
Detection of GFP-DMinC and GFP-DicB by immunoblotting. Immunoblots containing whole-cell extracts were probed with anti-GFP antibodies. a to c, positions of GFP-DMinC (a) and both full-length (b) and processed (c) forms of GFP-DicB. The positions of molecular mass standards (in kilodaltons) and of an unidentified cross-reacting antigen that is present in all extracts (∗) are indicated on the left. Lanes contained extracts of strains PB114 [Δ_minCDE_] (lane 1), PB114(λDB182)/pLL13 [Δ_minCDE_(Plac::dicB)/c_I857, PλR::gfp-minC (14-231)] (lanes 2 and 3), and PB114(λJE39)/pJE46 [Δ_minCDE(Plac::gfp-dicB)/_c_I857, PλR::minC(14-231)] (lanes 4 and 5). Cells were grown at 30°C (lane 4) or 37°C (lanes 1 to 3 and 5) in the presence of 0 (lane 2), 37 (lanes 4 and 5), or 100 (lanes 1 and 3) μM IPTG.
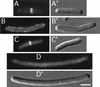
FIG. 5.
DicB- and MinD-mediated targeting of DMinC to septal rings. Micrographs showing colocalization of GFP-DMinC (A and B) and FtsZ (A" and B") in cells expressing either DicB (A) or MinD (B). Cells of strains PB114(λDB182)/pLL13 [Δ_minCDE_(Plac::dicB)/c_I857, PλR::gfp-minC(14-231)] (A) and PB114(λDR155)/pLL13 [Δ_minCDE(Plac::minD)/_c_I857, PλR::gfp-minC(14-231)] (B) were grown at 37°C in the presence of 100 μM IPTG, chemically fixed, and subjected to immunostaining with anti-FtsZ primary antibodies and Cy3-conjugated secondary antibodies. Cells were viewed through Nomarski (A′ and B′) and fluorescence optics with GFP-specific (A and B) or Cy3-specific (A" and B") filter sets. Bar, 2 μm.
FIG. 6.
Localization of DMinC in SfiA-induced filaments. Micrographs showing the location of GFP-DMinC in MinD+ (A and B) and DicB+ (C and D) cells in the absence (A and C) or presence (B and D) of SfiA. Strains used were PB114(λDR155)/pLL13/pBAD33 [Δ_minCDE_(Plac::minD)/c_I857, PλR::gfp-minC(14-231)/vector] (A), PB114(λDR155)/pLL13/pJE80 [Δ_minCDE(Plac::minD)/_c_I857, PλR::gfp-minC(14-231)/PBAD::sfiA_] (B), PB114(λDB182)/pLL13/pBAD33 [Δ_minCDE(Plac::dicB)/c_I857, PλR::gfp-minC(14-231)/vector] (C), and PB114(λDB182)/pLL13/pJE80 [Δ_minCDE(Plac::dicB)/_c_I857, PλR::gfp-minC(14-231)/PBAD::_sfiA_] (D). Cells were grown at 37°C in the presence of 100 μM IPTG to an OD600 of 0.1, arabinose was added to 0.1%, and growth was allowed to continue for 45 min before inspection by fluorescence (A to D) and DIC (A′ to D′) microscopy. Bar, 2 μm.
FIG. 7.
Mutually dependent accumulation of DMinC and MinD on rings. Micrographs show the distribution of GFP-DMinC (A to D) in the absence (A) and presence (B to D) of MinD and that of GFP-MinD in the absence (E) and presence (F) of DMinC. (A to D) Cells of strain PB114(λDR155)/pLL13 [Δ_minCDE_(Plac::minD)/c_I857, PλR::gfp-minC(14-231)] which were grown at 37°C in the absence (A) or presence (B to D) of 100 μM IPTG. (E and F) Cells of strain PB114(λDR119)/pJE46 [Δ_minCDE(Plac::gfp-minD)/_c_I857, PλR::minC (14-231)] which were grown in the presence of 37 μM IPTG at 30°C (E) or 37°C (F). Bar, 2 μm.
FIG. 8.
DicB removes MinD from rings. Micrographs show the distribution of GFP-MinD in the presence of DMinC and in either the absence (A) or presence (B and C) of DicB. Cells of strain PB114(λDR119)/pJE46/pJE44 [Δ_minCDE_(Plac::gfp-minD)/_c_I857, PλR::minC (14-231)/PBAD::_dicB_] were grown at 37°C in the presence of 37 μM IPTG, and no (A), 0.02% (B), or 0.05% (C) arabinose. Bar, 2 μm.
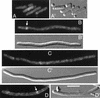
FIG. 9.
Transient association of oscillating DMinC with rings. Time-lapse images show the oscillation of GFP-MinC (A) and GFP-DMinC (B and C) in the presence of MinD and MinE. Shown are cells of strain LL1(λDB175) [Δ_minCDE_(Plac::minDE)] harboring either pLL18 [_c_I857, PλR::gfp-minC(5-231)] (A) or pPC105 [_c_I857, PλR::gfp-minC(108-231)] (B and C). Cells were grown at 37°C in the presence of 100 μM IPTG. Times in seconds are indicated. Arrows in the DIC panels (B and C) mark the positions of rings to which GFP-DMinC transiently associated as it moved from one end of the cell to the other. Such transient associations are not observed when the Z domain of MinC is functional (A). Bar, 2 μm.
FIG. 10.
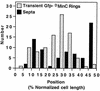
Positions of rings transiently decorated by oscillating DMinC. Time-lapse and DIC images of randomly chosen cells of strain LL1(λDB175)/pPC105 (see legend to Fig. 9) were collected, and the positions of transiently fluorescent ring structures and division septa were determined. x axis, position of structures relative to the proximal cell pole and midcell after normalization of cell length; y axis, number of structures at each position. The distributions of transiently fluorescent rings and septa represent data from time-lapse images of 77 live cells and DIC images of 260 fixed cells, respectively. Data from cells >7.0 μm were not included because the majority of these showed a multizonal oscillation pattern, similar to that described before (46, 48), rather than the pole-to-pole pattern seen in smaller cells.
FIG. 11.
GFP-DMinC/DicB rings are resistant to MinE. Fluorescence (A to D) and DIC (A′ to D′) micrographs show the localization of GFP-DMinC in MinD+ DicB− (A and B) and MinD− DicB+ (C and D) cells in the absence (A and C) or upon (over)expression (B and D) of MinE. Shown are cells of strains PB114(λDR155)/pLL13/pBAD33 [Δ_minCDE_(Plac::minD)/c_I857, PλR::gfp-minC (14-231)/vector] (A), PB114(λDR155)/pLL13/pJE75 [Δ_minCDE(Plac::minD)/_c_I857, PλR::gfp-minC (14-231)/PBAD::minE_] (B), PB114(λDB182)/pLL13/pBAD33 [Δ_minCDE(Plac::dicB)/c_I857, PλR::gfp-minC(14-231)/vector] (C), and PB114(λDB182)/pLL13/JE75 [Δ_minCDE(Plac::dicB)/_c_I857, PλR::gfp-minC (14-231)/PBAD::_minE_] (D). Cells were grown for 3.5 h to an OD600 of 0.3 at 37°C in the presence of 100 μM IPTG and 0.1% arabinose before examination. Bar, 2 μm.
FIG. 12.
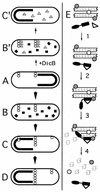
Models for MinD- and DicB-dependent activation of MinC-mediated division inhibition. Under conditions of normal growth (A to D), MinC and MinD co-oscillate from the membrane on one cell half to the other in a MinE-dependent fashion (MinC/MinD is indicated by a thick black line or squares; MinE is omitted for simplicity). FtsZ polymerization (chains of open circles) is allowed to initiate on any site of the membrane not occupied by MinC/MinD and not subject to nucleoid occlusion (38). As soon as MinC/MinD returns to occupy that half of the membrane, nascent ring structures that formed in the previous half cycle and that are positioned off-center are the substrate for MinC/MinD-mediated destruction (C and D), while structures that may have formed at the cell center are protected because the oscillating behavior of the division inhibitor causes its time-averaged concentration to be minimal at the cell's middle (22, 25, 41). Upon induction of dicB expression in WT cells (B′ and C′), DicB efficiently competes with MinD for binding MinC to form MinC/DicB complexes (shaded triangles). The latter do not oscillate and destroy nascent septal ring structures at all sites in the cell, resulting in filamentous growth of the bacterium. At a molecular level (E), MinC-mediated division inhibition in vivo is likely to occur in several steps. In step 1, MinD or DicB (open triangle) binds the C-terminal D domain of MinC (DMinC; black rectangle portion of molecule). This binding event produces a complex with a high affinity for a target that is part of both nascent and mature septal ring structures. In the simplest scenario, the inhibitor complex directly recognizes polymers of FtsZ (chain of Z-shaped monomers). Alternatively, additional septal ring components (open and shaded circles) may first need to associate with FtsZ polymers to create target sites. In step 2, DMinC/MinD or DMinC/DicB binds the target. This event is independent of any interaction involving the N-terminal domain of MinC (ZMinC; black oval portion of molecule). Rather, step 2 serves to bring ZMinC in close proximity to its substrate, allowing efficient disassembly of FtsZ polymers in step 3. Disassembly of FtsZ polymers by ZMinC simultaneously destroys the septal ring ligand(s) recognized by the DMinC complex (step 4). As a result, MinC/DicB is instantly released into the cytoplasm, whereas MinC/MinD remains associated with the surrounding membrane until it is forced off by the action of the next MinE wave (15, 22, 27).
Similar articles
- ZipA is required for targeting of DMinC/DicB, but not DMinC/MinD, complexes to septal ring assemblies in Escherichia coli.
Johnson JE, Lackner LL, Hale CA, de Boer PA. Johnson JE, et al. J Bacteriol. 2004 Apr;186(8):2418-29. doi: 10.1128/JB.186.8.2418-2429.2004. J Bacteriol. 2004. PMID: 15060045 Free PMC article. - MinC mutants deficient in MinD- and DicB-mediated cell division inhibition due to loss of interaction with MinD, DicB, or a septal component.
Zhou H, Lutkenhaus J. Zhou H, et al. J Bacteriol. 2005 Apr;187(8):2846-57. doi: 10.1128/JB.187.8.2846-2857.2005. J Bacteriol. 2005. PMID: 15805531 Free PMC article. - MinC N- and C-Domain Interactions Modulate FtsZ Assembly, Division Site Selection, and MinD-Dependent Oscillation in Escherichia coli.
LaBreck CJ, Conti J, Viola MG, Camberg JL. LaBreck CJ, et al. J Bacteriol. 2019 Jan 28;201(4):e00374-18. doi: 10.1128/JB.00374-18. Print 2019 Feb 15. J Bacteriol. 2019. PMID: 30455283 Free PMC article. - The E. coli MinCDE system in the regulation of protein patterns and gradients.
Ramm B, Heermann T, Schwille P. Ramm B, et al. Cell Mol Life Sci. 2019 Nov;76(21):4245-4273. doi: 10.1007/s00018-019-03218-x. Epub 2019 Jul 17. Cell Mol Life Sci. 2019. PMID: 31317204 Free PMC article. Review. - Assembly dynamics of the bacterial cell division protein FTSZ: poised at the edge of stability.
Romberg L, Levin PA. Romberg L, et al. Annu Rev Microbiol. 2003;57:125-54. doi: 10.1146/annurev.micro.57.012903.074300. Annu Rev Microbiol. 2003. PMID: 14527275 Free PMC article. Review.
Cited by
- A newly identified prophage-encoded gene, ymfM, causes SOS-inducible filamentation in Escherichia coli.
Ansari S, Walsh JC, Bottomley AL, Duggin IG, Burke C, Harry EJ. Ansari S, et al. J Bacteriol. 2021 Jun 1;203(11):e00646-20. doi: 10.1128/JB.00646-20. Epub 2021 Mar 15. J Bacteriol. 2021. PMID: 33722843 Free PMC article. - Interaction between cell division proteins FtsE and FtsZ.
Corbin BD, Wang Y, Beuria TK, Margolin W. Corbin BD, et al. J Bacteriol. 2007 Apr;189(8):3026-35. doi: 10.1128/JB.01581-06. Epub 2007 Feb 16. J Bacteriol. 2007. PMID: 17307852 Free PMC article. - YdfD, a Lysis Protein of the Qin Prophage, Is a Specific Inhibitor of the IspG-Catalyzed Step in the MEP Pathway of Escherichia coli.
Lu Z, Wang B, Qiu Z, Zhang R, Zheng J, Jia Z. Lu Z, et al. Int J Mol Sci. 2022 Jan 29;23(3):1560. doi: 10.3390/ijms23031560. Int J Mol Sci. 2022. PMID: 35163484 Free PMC article. - The C-terminal domain of MinC inhibits assembly of the Z ring in Escherichia coli.
Shiomi D, Margolin W. Shiomi D, et al. J Bacteriol. 2007 Jan;189(1):236-43. doi: 10.1128/JB.00666-06. Epub 2006 Nov 3. J Bacteriol. 2007. PMID: 17085577 Free PMC article. - Roles of the DedD Protein in Escherichia coli Cell Constriction.
Liu B, Hale CA, Persons L, Phillips-Mason PJ, de Boer PAJ. Liu B, et al. J Bacteriol. 2019 Mar 26;201(8):e00698-18. doi: 10.1128/JB.00698-18. Print 2019 Apr 15. J Bacteriol. 2019. PMID: 30692172 Free PMC article.
References
- Addinall, S. G., and J. Lutkenhaus. 1996. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol. 22:231-237. - PubMed
- Béjar, S., F. Bouché, and J.-P. Bouché. 1988. Cell division inhibition gene dicB is regulated by a locus similar to lambdoid bacteriophage immunity loci. Mol. Gen. Genet. 212:11-19. - PubMed
- Bi, E., and J. Lutkenhaus. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161-164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases