Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation - PubMed (original) (raw)
Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation
Eric W Hewitt et al. EMBO J. 2002.
Abstract
The Kaposi's sarcoma-associated herpes virus gene product K3 (KK3) subverts the MHC class I antigen presentation pathway by downregulating MHC class I from the plasma membrane. We now show that KK3 associates with MHC class I molecules and promotes ubiquitylation of class I after export from the endoplasmic reticulum. Ubiquitylation requires the KK3 N-terminal plant homeodomain and provides the signal for class I internalization at the plasma membrane. Once internalized, ubiquitylated MHC class I is targeted to the late endocytic pathway, where it is degraded. Depletion by small interfering RNA of TSG101, a ubiquitin enzyme 2 variant protein involved in late endosomal sorting, prevents class I degradation and preserves cell surface class I expression in KK3-expressing cells. These results suggest a mechanism by which the KK3-induced class I ubiquitylation provides a signal for both internalization and sorting to the late endosomal pathway for degradation. KK3 is the first viral gene product that subverts the trafficking of a host protein via the ubiquitin-dependent endosomal sorting machinery.
Figures
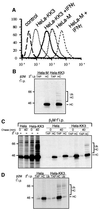
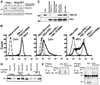
Fig. 1. KK3 promotes the ubiquitylation of MHC class I heavy chains. (A) Cytofluorometric analysis of MHC class I expression in HeLa-M and HeLa-KK3 cells. HeLa-M and HeLa-KK3 cells were cultured for 24 h in the absence or presence of 200 U/ml IFN-γ and stained with the mAb W6/32 and a FITC-conjugated secondary antibody. (B) High molecular weight laddering of class I molecules in KK3-expressing cells. Hela-M or Hela-KK3 cells (2 × 107) were pulse-labelled for 15 min and chased for 40 min. Following 1% Triton X-100 detergent solubilization, MHC class I molecules were immunoprecipitated with a β2M-specific antiserum, and after dissociation in 1% SDS, re-precipitated with either the TAP2-specific antibody 435.3 or the heavy chain- specific antibody HC10, and resolved on a 10% SDS–PAGE gel prior to autoradiography. (C and D) The high molecular weight laddering following class I immunoprecipitations in KK3-expressing cells represents ubiquitylated class I molecules. (C) IFN-γ-stimulated Hela-M and Hela-KK3 cells (1.2 × 107) were pulse-labelled for 15 min and chased for 0 or 40 min. Following solubilization in Triton X-100, MHC class I molecules were immunoprecipitated with a β2M-specific antiserum. One-third of the β2M immunoprecipitate was resolved on a 10% SDS–PAGE gel, and the remainder dissociated in 1% SDS and re-precipitated with either the TAP2-specific mAb 435.3 or the heavy chain- specific mAb HC10. (D) IFN-γ-stimulated cells (6 × 106) were pulse-labelled as in (C), but following dissociation in 1% SDS, lysates were re-precipitated with either the TAP2-specific mAb 435.3, the heavy chain-specific mAb HC10 or the ubiquitin mAb FK2.
Fig. 2. Ubiquitylated class I molecules are glycosylated, resistant to Endo H digestion and ubiquitylation is inhibited by BFA. (A) Ubiquitylated class I molecules are Endo H resistant and Endo F sensitive. IFN-γ-treated Hela-M and Hela-KK3 cells (107) were pulse-labelled for 30 min and chased for 40 min. MHC class I molecules were immunoprecipitated from Triton X-100 lysates with a β2M- specific antiserum, dissociated in 1% SDS and re-precipitated with either the TAP2-specific mAb 435.3 or the heavy chain-specific mAb HC10. The HC10 re-precipitate was either directly resolved, digested (+) or mock digested (–) with Endo H or Endo F prior to SDS–PAGE. (B) In the presence of BFA, MHC class I ubiquitylation is abolished. Hela-M and Hela-KK3 cells were pulse-labelled as in (A), but in the presence or absence of 4 µg/ml BFA. MHC class I molecules were immunoprecipitated with a β2M-specific antiserum, dissociated in 1% SDS and re-precipitated with either the TAP2-specific mAb 435.3 or the heavy chain-specific mAb HC10.
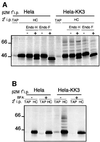
Fig. 3. KK3 associates with and promotes ubiquitylation of MHC class I molecules. (A) KK3 associates with MHC class I molecules. IFN-γ-stimulated Hela-M and Hela-KK3 cells (2 × 107) were pulse- labelled for 12 min and chased for 40 min. The KK3 viral protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2. Ten percent of the FLAG mAb immunoprecipitate was directly resolved on a 10% SDS–PAGE gel (left panel), and the remainder dissociated in 1% SDS and re-precipitated with the heavy chain-specific mAb HC10 and the FLAG mAb M2 (for KK3) (middle panel). The experiment was repeated with HeLa-KK3 cells and, following SDS dissociation, was re-precipitated with the same two mAbs as well as the ubiquitin (Ub)-specific mAb FK2 (right panel). (B) The KK3 interaction with MHC class molecules I is detergent sensitive. IFN-γ-stimulated Hela-KK3 cells were pulse-labelled as in (A) and extracted in 1% digitonin. After removal of the nuclear fraction, the lysate was divided into three aliquots. Triton X-100 or DOC (1% final) was added to each of two aliquots. Cell lysates were immunoprecipitated for the KK3 protein with the FLAG mAb M2, dissociated in 1% SDS and re-precipitated with either the heavy chain-specific mAb HC10 (left panel) or the FLAG mAb M2 (for KK3) (right panel).
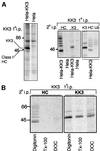
Fig. 4. KK3-induced ubiquitylation of MHC class I molecules occurs in a post-ER compartment. (A and B) KK3 associates with Endo H-resistant class I molecules. (A) IFN-γ-stimulated Hela-KK3 cells (2.8 × 107) were pulse-labelled for 5 min and chased for the indicated time periods. The KK3 protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2, digested (+) or mock digested (–) with Endo H prior to resolution by SDS–PAGE and subsequent autoradiography. To control for Endo H activity, MHC class I molecules were immunoprecipitated from Triton X-100 lysates with the β2M- specific antiserum and subjected to Endo H digestion as above (right panel). (B) HeLa-KK3 cells were pulse-labelled as in (A) for the indicated time periods, and the KK3 protein immunoprecipitated from digitonin lysates with the FLAG mAb M2. Following dissociation in 1% SDS, lysates were re-precipitated with either the heavy chain- specific mAb (HC10) (upper panel) or the FLAG mAb M2 (for KK3) (lower panel), and resolved on a 10% SDS–PAGE gel. (C) Bafilomycin prevents degradation of KK3-associated ubiquitylated class I molecules. HeLa-KK3 cells were pulse-labelled as in (A) and chased for 3 h in the presence (+) or absence (–) of bafilomycin. The KK3 protein was immunoprecipitated from digitonin lysates with the FLAG mAb M2, dissociated in 1% SDS, and lysates re-precipitated with the heavy chain-specific mAb (HC10). (D) BFA inhibits the association between KK3 and class I. HeLa-KK3 cells were pulse-labelled as in (A) and chased for 1 h in the presence (+) or absence (–) of BFA and lactacystin (Lacta.). The KK3 protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2, dissociated in 1% SDS, and lysates were re-precipitated with either the heavy chain-specific antibody (HC10) or the FLAG mAb M2 (for KK3), and resolved on a 10% SDS–PAGE gel.
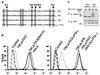
Fig. 5. Mutation of the PHD motif of KK3 preserves cell surface class I expression by preventing ubiquitylation but not class I association. (A) Sequence alignment of the N-terminal PHD motifs of KK3, KK5 and MK3 with the RING domain of c-Cbl. The canonical cysteine and histidine residues are boxed. Residues mutated in this study are indicated with arrows. (B) Cytofluorometric analysis of cell surface MHC class I expression in HeLa-M cells stably transduced with wild-type or mutant (W41A) KK3. IFN-γ-stimulated (right panel) or non-stimulated (left panel) cells were stained with the mAb W6/32 and a FITC-conjugated secondary antibody. Control cells were only stained with the secondary antibody. (C) The KK3(W41A) PHD domain mutant preserves the association of KK3 with class I, but is not ubiquitylated. IFN-γ-stimulated HeLa-M cells (1 × 107) stably transduced with wild-type (WT) or mutant (W41A) KK3 were pulse-labelled for 5 min and chased for 60 and 180 min, as indicated. The KK3 protein was immunoprecipitated from digitonin cell lysates with the FLAG mAb M2, dissociated in 1% SDS and lysates re-precipitated with either the heavy chain-specific mAb (HC10) (upper panel) or the FLAG mAb M2 (for KK3) (lower panel).
Fig. 6. A cytoplasmic tail lysine residue is required for the internalization of HLA-A2 mediated by KK3. (A) Sequence alignment of the cytoplasmic tails of HLA-A2, HLA-B2705, HLA-Cw2 and HLA-E. The stop transfer sequence is shown in italics; lysine residues in the cytoplasmic tail are boxed with K340 and K364 corresponding to the HLA-A2 sequence. (B) Cytofluorometric analysis of MHC class I expression in HeLa (upper six panels) and HeLa-KK3 cells (lower six panels) transiently transfected with either wild-type HLA-A2 or the lysine mutants HLA-A2-K340A, HLA-A2 K364A and HLA-A2 K340A/K364A. Transfected cells were stained with the mAb BB7.2 and a FITC-conjugated secondary antibody. (C) Internalization of HLA-A2. HeLa-M (i, iii, v and vii) and HeLa-KK3 cells (ii, iv, vi and viii) were transiently transfected with either wild-type HLA-A2 (i and ii) or the lysine mutants HLA-A2-K340A (iii and iv), HLA-A2 K364A (v and vi) and HLA-A2 K340A/K364A (vii and viii). The HLA-A2-specific mAb BB7.2 was bound to the transfectants on ice for 1 h and the cells subsequently incubated at 37°C for 30 min before fixation. BB7.2 staining was detected with an anti-mouse FITC antibody and visualized with a Zeiss Axioplan microscope. The scale bar is 10 µm.
Fig. 7. Cellular depletion of the TSG101 protein inhibits KK3-induced MHC class I degradation. (A) Depletion of TSG101 from HeLa-M and HeLa-KK3 using siRNA. Cells were co-transfected twice with 50 nM RNAi (lanes 2 and 5) or mock transfected (lanes 1 and 4) at 24 h intervals. Forty-eight hours after the first transfection, 2 × 105 cells were extracted in 1% Triton X-100, and lysates separated by 10% SDS–PAGE, transferred to Immobilon P membranes, and probed with the A410 anti-TSG101-specific mAb (upper panel) or control anti-calnexin antisera (lower panel). (B) Cytofluorometric analysis of cell surface MHC class I expression in HeLa-M and HeLa-KK3 cells following TSG101 depletion. Cells (2 × 105) were stained with the monoclonal mAb W6/32 and a FITC-conjugated secondary antibody at 24 h (left and middle panel) and 48 h (right panel) post-TSG101RNAi transfection. (C) TSG101-depleted HeLa-KK3 cells were sorted at the 48 h time point [as in (B), right panel] into class I ‘high’ and ‘low’ populations and probed with the A410 anti-TSG101-specific mAb (upper panel) or FLAG mAb M2 (for KK3) (lower panel). (D) TSG101 depletion prevents class I degradation. HeLa-M or Hela-KK3 cells (5 × 105) were pulse-labelled for 10 min and chased for 3 h. Triton X-100 cell lysates were immunoprecipitated with the β2M antiserum and resolved by SDS–PAGE and subsequent autoradiography. (E) Class I remains ubiquitylated in TSG101-depleted KK3 cells. IFN-γ-stimulated HeLa-KK3 or TSG101-depleted HeLa-KK3 cells (1.4 × 107) were pulse-labelled for 8 min and chased for 60–180 min. The KK3 protein was immunoprecipitated with the FLAG mAb M2, dissociated in 1% SDS and lysates re-precipitated with the heavy chain-specific mAb (HC10) (left panel). Proteins not precipitated with the FLAG mAb were re-precipitated with the rabbit β2M-specific antiserum, dissociated in 1% SDS and re-precipitated with the heavy chain-specific mAb HC10 (right panel), and resolved on a 10% SDS–PAGE gel. The 29 kDa band represents a class I degradation product.
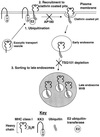
Fig. 8. Model for the downregulation of MHC class I by KK3. (1) KK3 promotes the ubiquitylation of MHC class I molecules that have trafficked through the secretory pathway at least as far as the _medial_-Golgi. For simplicity, the plasma membrane is represented as the site of ubiquitylation. KK3 presumably recruits via its PHD motif an E2 ubiquitin-transferase facilitating ubiquitylation of the MHC class I cytoplasmic tail. (2) Ubiquitylation recruits the MHC class I molecule into clathrin-coated pits and the class I is internalized into early endosomes. This step can be inhibited by overexpression of AP180. (3) Once within the endosomal pathway, TSG101-dependent sorting targets the ubiquitylated MHC class I molecule to late endosomes. Assuming conservation of the yeast and mammalian MVB sorting machinery, we propose that ubiquitin sorts the MHC class I molecules into the internal vesicles in the MVB.
Similar articles
- TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation.
Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. Lu Q, et al. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7626-31. doi: 10.1073/pnas.0932599100. Epub 2003 Jun 11. Proc Natl Acad Sci U S A. 2003. PMID: 12802020 Free PMC article. - Ubiquitylation of the gap junction protein connexin-43 signals its trafficking from early endosomes to lysosomes in a process mediated by Hrs and Tsg101.
Leithe E, Kjenseth A, Sirnes S, Stenmark H, Brech A, Rivedal E. Leithe E, et al. J Cell Sci. 2009 Nov 1;122(Pt 21):3883-93. doi: 10.1242/jcs.053801. Epub 2009 Oct 6. J Cell Sci. 2009. PMID: 19808888 - [The HIV nef and the Kaposi-sarcoma-associated virus K3/K5 proteins: "parasites"of the endocytosis pathway].
Bénichou S, Benmerah A. Bénichou S, et al. Med Sci (Paris). 2003 Jan;19(1):100-6. doi: 10.1051/medsci/2003191100. Med Sci (Paris). 2003. PMID: 12836198 Review. French. - Constitutive internalization of murine MHC class I molecules.
Mahmutefendić H, Blagojević G, Kucić N, Lucin P. Mahmutefendić H, et al. J Cell Physiol. 2007 Feb;210(2):445-55. doi: 10.1002/jcp.20877. J Cell Physiol. 2007. PMID: 17044074 - Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses.
Früh K, Bartee E, Gouveia K, Mansouri M. Früh K, et al. Virus Res. 2002 Sep;88(1-2):55-69. doi: 10.1016/s0168-1702(02)00120-x. Virus Res. 2002. PMID: 12297327 Review.
Cited by
- Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes?
Nathan JA, Kim HT, Ting L, Gygi SP, Goldberg AL. Nathan JA, et al. EMBO J. 2013 Feb 20;32(4):552-65. doi: 10.1038/emboj.2012.354. Epub 2013 Jan 11. EMBO J. 2013. PMID: 23314748 Free PMC article. - Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR.
Wang Y, Huang Y, Hobbs HH, Cohen JC. Wang Y, et al. J Lipid Res. 2012 Sep;53(9):1932-43. doi: 10.1194/jlr.M028563. Epub 2012 Jul 4. J Lipid Res. 2012. PMID: 22764087 Free PMC article. - The Membrane Associated RING-CH Proteins: A Family of E3 Ligases with Diverse Roles through the Cell.
Samji T, Hong S, Means RE. Samji T, et al. Int Sch Res Notices. 2014 Oct 29;2014:637295. doi: 10.1155/2014/637295. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27419207 Free PMC article. Review. - Requirements for the selective degradation of endoplasmic reticulum-resident major histocompatibility complex class I proteins by the viral immune evasion molecule mK3.
Wang X, Connors R, Harris MR, Hansen TH, Lybarger L. Wang X, et al. J Virol. 2005 Apr;79(7):4099-108. doi: 10.1128/JVI.79.7.4099-4108.2005. J Virol. 2005. PMID: 15767411 Free PMC article. - Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II.
Durrington HJ, Upton PD, Hoer S, Boname J, Dunmore BJ, Yang J, Crilley TK, Butler LM, Blackbourn DJ, Nash GB, Lehner PJ, Morrell NW. Durrington HJ, et al. J Biol Chem. 2010 Nov 26;285(48):37641-9. doi: 10.1074/jbc.M110.132415. Epub 2010 Sep 24. J Biol Chem. 2010. PMID: 20870717 Free PMC article.
References
- Aasland R., Gibson,T.J. and Stewart,A.F. (1995) The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci., 20, 56–59. - PubMed
- Babst M., Odorizzi,G., Estepa,E.J. and Emr,S.D. (2000) Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic, 1, 248–258. - PubMed
- Boname J.M. and Stevenson,P.G. (2001) MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity, 15, 627–636. - PubMed
- Cesarman E. and Knowles,D.M. (1999) The role of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin. Cancer Biol., 9, 165–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials