Expression of the biofilm-associated protein interferes with host protein receptors of Staphylococcus aureus and alters the infective process - PubMed (original) (raw)
Expression of the biofilm-associated protein interferes with host protein receptors of Staphylococcus aureus and alters the infective process
Carme Cucarella et al. Infect Immun. 2002 Jun.
Abstract
The adherence of Staphylococcus aureus to soluble proteins and extracellular-matrix components of the host is one of the key steps in the pathogenesis of staphylococcal infections. S. aureus presents a family of adhesins called MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that specifically recognize host matrix components. We examined the influence of biofilm-associated protein (Bap) expression on S. aureus adherence to host proteins, epithelial cell cultures, and mammary gland sections and on colonization of the mammary gland in an in vivo infection model. Bap-positive strain V329 showed lower adherence to immobilized fibrinogen and fibronectin than isogenic Bap-deficient strain m556. Bacterial adherence to histological sections of mammary gland and bacterial internalization into 293 cells were significantly lower in the Bap-positive strains. In addition, the Bap-negative strain showed significantly higher colonization in vivo of sheep mammary glands than the Bap-positive strain. Taken together, these results strongly suggest that the expression of the Bap protein interferes with functional properties of the MSCRAMM proteins, preventing initial bacterial attachment to host tissues and cellular internalization.
Figures
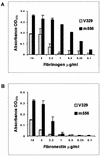
FIG. 1.
Adhesion of S. aureus V329 and m556 to immobilized fibrinogen (A) and fibronectin (B). Wells in ELISA plates were coated with different concentrations of human fibrinogen or fibronectin. Adherence of strain V329 and the Bap mutant m556 was indicated by staining with crystal violet and measuring the absorbance at 570 nm. Data are the average results ± standard deviations of triplicate determinations. Background values of bacteria adhering to BSA-coated wells were subtracted.
FIG. 2.
Adhesion of Bap-positive S. aureus Newman and P1 strains to immobilized fibrinogen (A) and fibronectin (B). Data are the average results ± standard deviations of triplicate determinations of the increase of bacterial adherence to uncoated versus protein-coated surfaces. (C) Biofilm formation of the complemented S. aureus Newman and P1 strains. ∗, P < 0.001 (t tests).
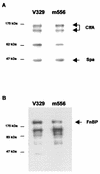
FIG. 3.
Visualization of ClfA protein (A) and FnBPs (B) by Western blotting and ligand affinity blots, respectively. Positions of protein size markers are shown at the left of each panel. Bands corresponding to native and truncated ClfA proteins, FnBPs, and Spa are identified. Similar amounts of ClfA, FnBPs, and protein A released into the supernatant by protoplasts stabilized in raffinose were observed in V329 and m556.
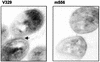
FIG. 4.
Ultrathin sections of Bap-positive and Bap-negative strains. Note the presence of extracellular polysaccharide material (arrow) stained with alcian blue in S. aureus V329, in contrast to what is seen in biofilm-negative mutant m556. Magnification, ×34,000.
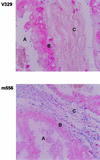
FIG. 5.
Adherence of S. aureus strains to a mammary gland section. Magnification, ×200. A, lumen of mammary gland; B, epithelial layer; C, connective tissue.
Similar articles
- Phase-variable expression of the biofilm-associated protein (Bap) in Staphylococcus aureus.
Tormo MÁ, Úbeda C, Martí M, Maiques E, Cucarella C, Valle J, Foster TJ, Lasa Í, Penadés JR. Tormo MÁ, et al. Microbiology (Reading). 2007 Jun;153(Pt 6):1702-1710. doi: 10.1099/mic.0.2006/005744-0. Microbiology (Reading). 2007. PMID: 17526828 - Bap, a biofilm matrix protein of Staphylococcus aureus prevents cellular internalization through binding to GP96 host receptor.
Valle J, Latasa C, Gil C, Toledo-Arana A, Solano C, Penadés JR, Lasa I. Valle J, et al. PLoS Pathog. 2012;8(8):e1002843. doi: 10.1371/journal.ppat.1002843. Epub 2012 Aug 2. PLoS Pathog. 2012. PMID: 22876182 Free PMC article. - The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen.
Wann ER, Gurusiddappa S, Hook M. Wann ER, et al. J Biol Chem. 2000 May 5;275(18):13863-71. doi: 10.1074/jbc.275.18.13863. J Biol Chem. 2000. PMID: 10788510 - Surface protein adhesins of Staphylococcus aureus.
Foster TJ, Höök M. Foster TJ, et al. Trends Microbiol. 1998 Dec;6(12):484-8. doi: 10.1016/s0966-842x(98)01400-0. Trends Microbiol. 1998. PMID: 10036727 Review. - Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus.
Hauck CR, Ohlsen K. Hauck CR, et al. Curr Opin Microbiol. 2006 Feb;9(1):5-11. doi: 10.1016/j.mib.2005.12.002. Epub 2006 Jan 6. Curr Opin Microbiol. 2006. PMID: 16406780 Review.
Cited by
- Preparation and optimization of niosome encapsulated meropenem for significant antibacterial and anti-biofilm activity against methicillin-resistant Staphylococcus aureus isolates.
Paseban K, Noroozi S, Gharehcheloo R, Haddadian A, Falahi Robattorki F, Dibah H, Amani R, Sabouri F, Ghanbarzadeh E, Hajrasouiha S, Azari A, Rashidian T, Mirzaie A, Pirdolat Z, Salarkia M, Shahrava DS, Safaeinikjoo F, Seifi A, Sadat Hosseini N, Saeinia N, Bagheri Kashtali A, Ahmadiyan A, Mazid Abadi R, Sadat Kermani F, Andalibi R, Chitgarzadeh A, Tavana AA, Piri Gharaghie T. Paseban K, et al. Heliyon. 2024 Aug 6;10(16):e35651. doi: 10.1016/j.heliyon.2024.e35651. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39211930 Free PMC article. - Detection of mecA Genes in Hospital-Acquired MRSA and SOSA Strains Associated with Biofilm Formation.
González-Vázquez R, Córdova-Espinoza MG, Escamilla-Gutiérrez A, Herrera-Cuevas MDR, González-Vázquez R, Esquivel-Campos AL, López-Pelcastre L, Torres-Cubillas W, Mayorga-Reyes L, Mendoza-Pérez F, Gutiérrez-Nava MA, Giono-Cerezo S. González-Vázquez R, et al. Pathogens. 2024 Feb 28;13(3):212. doi: 10.3390/pathogens13030212. Pathogens. 2024. PMID: 38535555 Free PMC article. - Immunoproteomic analysis of the serum IgG response to cell wall-associated proteins of Staphylococcus aureus strains belonging to CC97 and CC151.
Drumm SD, Cormican P, Owens RA, Mitchell J, Keane OM. Drumm SD, et al. Vet Res. 2023 Sep 18;54(1):79. doi: 10.1186/s13567-023-01212-7. Vet Res. 2023. PMID: 37723537 Free PMC article. - Biology and Regulation of Staphylococcal Biofilm.
François P, Schrenzel J, Götz F. François P, et al. Int J Mol Sci. 2023 Mar 9;24(6):5218. doi: 10.3390/ijms24065218. Int J Mol Sci. 2023. PMID: 36982293 Free PMC article. Review. - Clinical and molecular characteristics of Staphylococcus aureus isolated from Chinese children: association among the agr groups and genotypes, virulence genes and disease types.
Xu Y, Qian SY, Yao KH, Dong F, Song WQ, Sun C, Yang X, Zhen JH, Liu XQ, Lv Z-, Yang X. Xu Y, et al. World J Pediatr. 2021 Apr;17(2):180-188. doi: 10.1007/s12519-021-00421-4. Epub 2021 Mar 3. World J Pediatr. 2021. PMID: 33660136
References
- Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. - PubMed
- Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources