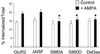Differential roles for NSF and GRIP/ABP in AMPA receptor cycling - PubMed (original) (raw)
Differential roles for NSF and GRIP/ABP in AMPA receptor cycling
Steven P Braithwaite et al. Proc Natl Acad Sci U S A. 2002.
Abstract
alpha-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) stability and movement at synapses are important factors controlling synaptic strength. Here, we study the roles of proteins [N-ethylmaleimide-sensitive fusion protein (NSF), glutamate receptor AMPAR binding protein (ABP)-interacting protein (GRIP)/(ABP), and protein interacting with C-kinase-1 (PICK1) that interact with the GluR2 subunit in the control of the surface expression and cycling of AMPARs. Epitope-tagged GluR2 formed functional receptors that exhibited targeting to synaptic sites. Constructs in which binding to NSF, PDZ proteins (GRIP/ABP and PICK1), or GRIP/ABP alone was eliminated each exhibited normal surface targeting and constitutive cycling. The lack of NSF binding, however, resulted in receptors that were endocytosed to a greater extent than wild-type receptors in response to application of AMPA or N-methyl-d-aspartate (NMDA). Conversely, the behavior of the GluR2 mutants incapable of binding to GRIP/ABP suggests that these PDZ proteins play a role in the stabilization of an intracellular pool of AMPARs that have been internalized on stimulation, thus inhibiting their recycling to the synaptic membrane. These results provide further evidence for distinct functional roles of GluR2-interacting proteins in AMPAR trafficking.
Figures
Figure 1
Expression and functional analysis of FLAG-GFP-GluR2. (a) Schematic drawing of FLAG-GFP-GluR2 indicating the binding sites within the C-terminal domain for NSF and PDZ domain containing proteins. (b) Representative electrophysiological responses from HEK293 cells transfected with FLAG-GFP-GluR2(Q). Cells were held at −70 mV and kainate (1 mM, Upper) or glutamate (1 mM, Lower) was applied. Scale bars = 100 pA, 100 ms. (c) Surface labeling (red) indicates that a proportion of the total FLAG-GFP-GluR2 (green) is delivered to the cell surface. (d) FLAG-GFP-GluR2 (green) colocalizes with synaptophysin (red) in hippocampal neuronal cultures.
Figure 2
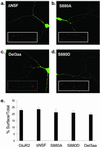
Surface expression of FLAG-GFP-GluR2 mutant constructs in cultured hippocampal neurons. (a_–_d) Examples of hippocampal neurons transfected with FLAG-GFP-GluR2 mutant constructs (green) all showing surface expressed receptors (red). (Insets) Higher power view of surface expressed puncta of FLAG-GFP-GluR2 mutant constructs. (a) FLAG-GFP-GluR2(ΔNSF). (b) FLAG-GFP-GluR2(S880A). (c) FLAG-GFP-GluR2(Del3aa). (d) FLAG-GFP-GluR2(S880D). (e) Quantification of the proportion of AMPARs on the cell surface compared with the total shows no difference between constructs (ANOVA: df = 4, MS = 79.5, F = 1.07, P = 0.37).
Figure 3
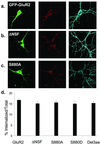
Constitutive endocytosis of FLAG-GFP-GluR2 is not influenced by binding to cytoplasmic proteins. (a_–_c) Representative images of transfected cells in which constitutive endocytosis of surface-expressed AMPARs was imaged. (Left) GFP fluorescence (green). (Center) Receptors that were surface labeled and then constitutively internalized (red). (Right) Remaining surface receptors (blue). (a) FLAG-GFP-GluR2. (b) FLAG-GFP-GluR2(ΔNSF). (c) FLAG-GFP-GluR2(S880A). (d) Quantification of the proportion of the total amount of surface-expressed AMPARs that were internalized shows no significant difference between constructs (ANOVA: df = 4, MS = 12.7, F = 0.60, P = 0.67).
Figure 4
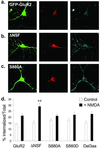
NMDA induces endocytosis of FLAG-GFP-GluR2 that is regulated by interaction with NSF. (a_–_c) Representative images of transfected cells in which endocytosis of AMPARs was triggered by NMDA application. Panels are the same as in Fig. 3. (a) FLAG-GFP-GluR2. (b) FLAG-GFP-GluR2(ΔNSF). (c) FLAG-GFP-GluR2(S880A). (d) Quantification shows all constructs exhibit significant internalization (P < 0.05) after NMDA stimulation (filled columns) compared with control conditions (open columns). There is significantly increased NMDA-induced internalization of FLAG-GFP-GluR2(ΔNSF) (**, P < 0.01) compared with FLAG-GFP-GluR2. There is no significant difference between constructs in the absence of NMDA (open columns). ANOVA: df = 4, MS = 64.7, F = 1.21, P = 0.31.
Figure 5
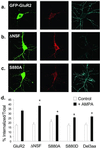
AMPA-induced endocytosis of FLAG-GFP-GluR2 is regulated by interaction with cytoplasmic proteins. (a_–_c) Representative images of transfected cells in which AMPA-induced endocytosis of FLAG-GFP-GluR2 constructs was imaged. Panels are the same as in Fig. 3. (a) FLAG-GFP-GluR2. (b) FLAG-GFP-GluR2(ΔNSF). (c) FLAG-GFP-GluR2(S880A). (d) Quantification shows all constructs exhibit significant internalization (P < 0.005) on AMPA stimulation (filled columns) compared with control conditions (open columns). There is significantly increased AMPA-induced internalization of FLAG-GFP-GluR2(ΔNSF) (*, P < 0.05) and decreased internalization of FLAG-GFP-GluR2(S880A), FLAG-GFP-GluR2(S880D), and FLAG-GFP-GluR2(Del3aa) compared with FLAG-GFP-GluR2 (*, P < 0.05). There is no significant difference between constructs in the absence of AMPA (open columns). ANOVA: df = 4, MS = 62.4, F = 0.98, P = 0.42.
Figure 6
The intracellular pool of internalized FLAG-GFP-GluR2 unable to interact with GRIP/ABP is dissipated 1 h after stimulation. One hour after AMPA treatment, FLAG-GFP-GluR2 and FLAG-GFP-GluR2(ΔNSF) still show a significant increase in the amount of internalized receptors compared with untreated cells (P < 0.05) whereas the mutants unable to interact with GRIP/ABP or PICK1 do not [FLAG-GFP-GluR2(S880A), P = 0.57; FLAG-GFP-GluR2(S880D), P = 0.25; FLAG-GFP-GluR2(Del3aa), P = 0.60). There is no significant difference in magnitude of AMPAR-induced internalization of FLAG-GFP-GluR2(ΔNSF) (P = 0.64), but decreased internalization of FLAG-GFP-GluR2(S880A), FLAG-GFP-GluR2(S880D), and FLAG-GFP-GluR2(Del3aa) compared with FLAG-GFP-GluR2 (*, P < 0.05). There is no significant difference between constructs in the absence of AMPA (open bars). ANOVA: df = 4, MS = 30.4, F = 0.61, P = 0.65).
Similar articles
- PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking.
Lu W, Ziff EB. Lu W, et al. Neuron. 2005 Aug 4;47(3):407-21. doi: 10.1016/j.neuron.2005.07.006. Neuron. 2005. PMID: 16055064 - PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization.
Lin DT, Huganir RL. Lin DT, et al. J Neurosci. 2007 Dec 12;27(50):13903-8. doi: 10.1523/JNEUROSCI.1750-07.2007. J Neurosci. 2007. PMID: 18077702 Free PMC article. - Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor.
Osten P, Khatri L, Perez JL, Köhr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Osten P, et al. Neuron. 2000 Aug;27(2):313-25. doi: 10.1016/s0896-6273(00)00039-8. Neuron. 2000. PMID: 10985351 - Regulation of neuronal PKA signaling through AKAP targeting dynamics.
Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Dell'Acqua ML, et al. Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review. - Interactions between AMPA receptors and intracellular proteins.
Braithwaite SP, Meyer G, Henley JM. Braithwaite SP, et al. Neuropharmacology. 2000 Apr 3;39(6):919-30. doi: 10.1016/s0028-3908(99)00171-9. Neuropharmacology. 2000. PMID: 10727702 Review.
Cited by
- Transcriptomic expression of AMPA receptor subunits and their auxiliary proteins in the human brain.
Shen K, Limon A. Shen K, et al. Neurosci Lett. 2021 Jun 11;755:135938. doi: 10.1016/j.neulet.2021.135938. Epub 2021 Apr 26. Neurosci Lett. 2021. PMID: 33915226 Free PMC article. Review. - Activity-dependent synaptic GRIP1 accumulation drives synaptic scaling up in response to action potential blockade.
Gainey MA, Tatavarty V, Nahmani M, Lin H, Turrigiano GG. Gainey MA, et al. Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):E3590-9. doi: 10.1073/pnas.1510754112. Epub 2015 Jun 24. Proc Natl Acad Sci U S A. 2015. PMID: 26109571 Free PMC article. - Time-Dependent Effects of Ethanol on BK Channel Expression and Trafficking in Hippocampal Neurons.
Palacio S, Velázquez-Marrero C, Marrero HG, Seale GE, Yudowski GA, Treistman SN. Palacio S, et al. Alcohol Clin Exp Res. 2015 Sep;39(9):1619-31. doi: 10.1111/acer.12808. Epub 2015 Aug 6. Alcohol Clin Exp Res. 2015. PMID: 26247146 Free PMC article. - Regulation of AMPA receptor trafficking and synaptic plasticity.
Anggono V, Huganir RL. Anggono V, et al. Curr Opin Neurobiol. 2012 Jun;22(3):461-9. doi: 10.1016/j.conb.2011.12.006. Epub 2012 Jan 2. Curr Opin Neurobiol. 2012. PMID: 22217700 Free PMC article. Review. - Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity.
Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, Wan Q, Liu F. Zou S, et al. J Neurosci. 2005 Apr 27;25(17):4385-95. doi: 10.1523/JNEUROSCI.5099-04.2005. J Neurosci. 2005. PMID: 15858065 Free PMC article.
References
- Malenka R C, Nicoll R A. Science. 1999;285:1870–1874. - PubMed
- Malinow R, Mainen Z F, Hayashi Y. Curr Opin Neurobiol. 2000;10:352–357. - PubMed
- Carroll R C, Beattie E C, Von Zastrow M, Malenka R C. Nat Rev Neurosci. 2001;2:315–324. - PubMed
- Geiger J R, Melcher T, Koh D S, Sakmann B, Seeburg P H, Jonas P, Monyer H. Neuron. 1995;15:193–204. - PubMed
- Wisden W, Seeburg P H. Curr Opin Neurobiol. 1993;3:291–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases