Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation - PubMed (original) (raw)
Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation
Andrew J Todd et al. J Neurosci. 2002.
Abstract
Lamina I of the spinal cord is densely innervated by nociceptive primary afferents, many of which contain substance P. It contains numerous projection neurons: the majority of these respond to noxious stimuli, however some are activated by cooling. In the rat, approximately 80% of the projection neurons express the neurokinin 1 (NK1) receptor, on which substance P acts, and most cells with this receptor are activated by noxious stimuli. Lamina I neurons can be classified morphologically into pyramidal, multipolar, and fusiform types. It has been reported in the cat that pyramidal neurons are activated only by cooling and that in monkey relatively few pyramidal cells are NK1 receptor-immunoreactive. We have used immunocytochemistry to examine the innervation of lamina I projection neurons in the rat by substance P-containing primary afferents and their responses to a noxious stimulus (subcutaneous formalin injection). NK1 receptor-immunoreactive projection cells received a significantly higher density of contacts from substance P-containing afferents than neurons that lacked the receptor. Most contacts on NK1 receptor-immunoreactive cells were associated with synapses. Formalin injection induced c-Fos in approximately 80% of projection neurons with the NK1 receptor and in 25-45% of those without it. More than 80% of pyramidal neurons expressed the receptor, and for both substance P innervation and c-Fos expression there were no significant differences among different morphological types of NK1 receptor-immunoreactive neuron. We conclude that presence or absence of the NK1 receptor is a better indicator of function than morphology for lamina I projection neurons in the rat.
Figures
Fig. 1.
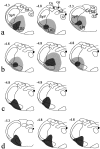
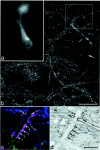
Diagrams to show the spread of tracer in each of the 11 experiments. The drawings in each _horizontal row_are from rats used for a single part of the study. _a,Fluorogold injections for confocal microscopic analysis of substance P–CGRP contacts on NK1 receptor-immunoreactive projection neurons;b, Fluorogold injections for combined confocal–electron microscopy of contacts; c, CTb injections for analysis of substance P contacts on projection neurons that lacked NK1 receptors; d, CTb injections for c-Fos experiments. For Fluorogold injections, the dark shaded area shows the necrotic core of the injection, and the paler area the halo of tracer, whereas for CTb injections, the shaded area shows the extent of CTb immunostaining in the injection site. In each case, the drawing shows the level of the medulla at which the maximum spread of tracer was present. Numbers at the_top left of each drawing give the approximate position of the section posterior to the ear bar. Drawings are based on those byPaxinos and Watson (1997). 12, Hypoglossal nucleus;Cu, cuneate nucleus; Gr, gracile nucleus;LRt, lateral reticular nucleus; Sol, tractus and nucleus solitarius; SpV, spinal trigeminal nucleus.
Fig. 2.
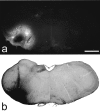
Examples of sections through injection sites with Fluorogold or CTb. a shows Fluorogold seen with epifluorescence illumination and an ultraviolet filter set, whereas_b_ is a section reacted to reveal CTb with an immunoperoxidase method. Scale bar, 1 mm.
Fig. 3.
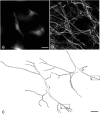
Examples of lamina I neurons retrogradely labeled with CTb and detected with immunofluorescence in a horizontal section. Note the large number of labeled cells and the extensive dendritic filling. Scale bar, 100 μm.
Fig. 4.
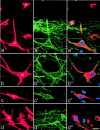
Two of the NK1 receptor-immunoreactive lamina I projection neurons included in the quantitative analysis of contacts from substance P primary afferents. a, Photograph of Fluorogold labeling in the two cells (1, 2) taken with an ultraviolet filter set. b, Confocal image of the corresponding field scanned to reveal NK1 receptor that is present on both cells and outlines their cell bodies and proximal dendrites. Cell 1 is a fusiform cell, and cell 2 is a pyramidal cell. Built from 11 optical sections at 1 μm _z_-spacing. _c,_Drawings of the two cells showing the contacts that they received from axonal varicosities that were immunoreactive with both substance P and CGRP antibodies. Each dot represents a single contact.Boxes show parts of the dendritic tree of each cell that are illustrated in Figure 5. Scale bars: a,b, 25 μm; c, 50 μm.
Fig. 5.
Confocal images showing contacts from substance P primary afferents onto the cell bodies and dendrites of the two NK1 receptor-immunoreactive lamina I projection neurons illustrated in Figure 4. In each row, the left image_shows NK1 receptor (green), the middle image shows substance P (red) and CGRP (blue), whereas in the right image all three colors have been merged. Profiles that contain both peptides appear purple. a and b_show the cell bodies of cells 1 and 2, whereas c and_d include parts of a dendrite from each cell (corresponding to boxes in Fig. 4_c). There are several contacts formed by axons that contain both substance P and CGRP on each cell body, and the dendrites receive so many contacts in these regions that they are outlined by immunoreactive axons. The images in a–d are built from projections of 6, 4, 5, and 4 optical sections, respectively, at 0.5 μm_z_-separation. Scale bar, 10 μm.
Fig. 6.
One of the multipolar cells examined with combined confocal and electron microscopy. a, Epifluorescence image taken with ultraviolet filter set to show Fluorogold in the cell body. b, Confocal image showing the NK1 receptor, which can be seen on the cell body and proximal dendrites (projected from 4 optical sections at 1 μm z_-separation). The_box shows the region illustrated in c and_d_. c, Confocal image showing a single optical section through the dendrite scanned to reveal NK1 receptor (green), substance P (red), and CGRP (blue). The dendrite is in contact with six axonal varicosities that contain both peptides and therefore appear_purple_ (numbered arrows).d, Low-magnification electron micrograph through the region corresponding to the confocal image in c. The six axonal varicosities are labeled with diaminobenzidine, which has been used to reveal substance P immunoreactivity, and can just be seen at this magnification. Scale bars: a, b, 25 μm; c, d, 10 μm.
Fig. 7.
High-magnification electron micrographs of the six substance P–CGRP-immunoreactive axonal varicosities illustrated in Figure 6_d_, taken either from the same ultrathin section or from one nearby in the series. In each case the labeled axon forms an asymmetrical synapse with the dendrite (D). The synaptic specialization is visible between the_arrows_. Numbers on the axons correspond to those in Figure 6, c and d. Scale bar, 0.5 μm.
Fig. 8.
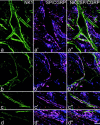
Expression of c-Fos in lamina I projection neurons. In each row, the _left panel_shows immunostaining for CTb (red) in a projected image from a confocal series, and the center panel shows the equivalent field scanned for NK1 receptor immunoreactivity (green). The right panel is a single optical section from the confocal series showing CTb (red), NK1 receptor (green), and c-Fos (blue). a, A CTb-labeled pyramidal cell (P) with the NK1 receptor has c-Fos in its nucleus, whereas another pyramidal neuron that lacks the receptor (N) is not c-Fos-immunoreactive.b, c, Multipolar (M) and fusiform (F) cells with the NK1 receptor have nuclear c-fos. d, This field contains several neurons, including one that is not NK1 receptor-immunoreactive (N) but has a nucleus that is c-Fos-immunoreactive. Projections in a–d are from seven, seven, five, and seven optical sections, respectively, at 2 μm _z_-separation. Scale bar, 20 μm.
Similar articles
- Fos induction in lamina I projection neurons in response to noxious thermal stimuli.
Todd AJ, Spike RC, Young S, Puskár Z. Todd AJ, et al. Neuroscience. 2005;131(1):209-17. doi: 10.1016/j.neuroscience.2004.11.001. Neuroscience. 2005. PMID: 15680704 - Substance P (NK1) and somatostatin (sst2A) receptor immunoreactivity in NTS-projecting rat dorsal horn neurones activated by nociceptive afferent input.
Gamboa-Esteves FO, McWilliam PN, Batten TF. Gamboa-Esteves FO, et al. J Chem Neuroanat. 2004 Jul;27(4):251-66. doi: 10.1016/j.jchemneu.2004.04.001. J Chem Neuroanat. 2004. PMID: 15261332 - Non-peptidergic primary afferents are presynaptic to neurokinin-1 receptor immunoreactive lamina I projection neurons in rat spinal cord.
Saeed AW, Ribeiro-da-Silva A. Saeed AW, et al. Mol Pain. 2012 Sep 10;8:64. doi: 10.1186/1744-8069-8-64. Mol Pain. 2012. PMID: 22963197 Free PMC article. - Selective innervation of lamina I projection neurones that possess the neurokinin 1 receptor by serotonin-containing axons in the rat spinal cord.
Polgár E, Puskár Z, Watt C, Matesz C, Todd AJ. Polgár E, et al. Neuroscience. 2002;109(4):799-809. doi: 10.1016/s0306-4522(01)00304-9. Neuroscience. 2002. PMID: 11927162
Cited by
- cAMP signaling through protein kinase A and Epac2 induces substance P release in the rat spinal cord.
Chen W, McRoberts JA, Ennes HS, Marvizon JC. Chen W, et al. Neuropharmacology. 2021 May 15;189:108533. doi: 10.1016/j.neuropharm.2021.108533. Epub 2021 Mar 17. Neuropharmacology. 2021. PMID: 33744339 Free PMC article. - Localization of neurones expressing the gap junction protein Connexin45 within the adult spinal dorsal horn: a study using Cx45-eGFP reporter mice.
Chapman RJ, Lall VK, Maxeiner S, Willecke K, Deuchars J, King AE. Chapman RJ, et al. Brain Struct Funct. 2013 May;218(3):751-65. doi: 10.1007/s00429-012-0426-1. Epub 2012 May 26. Brain Struct Funct. 2013. PMID: 22638825 Free PMC article. - Aberrant synaptic integration in adult lamina I projection neurons following neonatal tissue damage.
Li J, Kritzer E, Craig PE, Baccei ML. Li J, et al. J Neurosci. 2015 Feb 11;35(6):2438-51. doi: 10.1523/JNEUROSCI.3585-14.2015. J Neurosci. 2015. PMID: 25673839 Free PMC article. - Intrathecal P/Q- and R-type calcium channel blockade of spinal substance P release and c-Fos expression.
Terashima T, Xu Q, Yamaguchi S, Yaksh TL. Terashima T, et al. Neuropharmacology. 2013 Dec;75:1-8. doi: 10.1016/j.neuropharm.2013.06.018. Epub 2013 Jun 26. Neuropharmacology. 2013. PMID: 23810829 Free PMC article. - Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: an immunofluorescence colocalization study.
Marvizón JC, Chen W, Murphy N. Marvizón JC, et al. J Comp Neurol. 2009 Nov 1;517(1):51-68. doi: 10.1002/cne.22130. J Comp Neurol. 2009. PMID: 19711397 Free PMC article.
References
- Bester H, Chapman V, Besson J-M, Bernard J-F. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol. 2000;83:2239–2259. - PubMed
- Bleazard L, Hill RG, Morris R. The correlation between the distribution of NK1 receptor and the actions of tachykinin agonists in the dorsal horn of the rat indicates that substance P does not have a functional role on substantia gelatinosa (lamina II) neurons. J Neurosci. 1994;14:7655–7664. - PMC - PubMed
- Broman J, Anderson S, Ottersen OP. Enrichment of glutamate-like immunoreactivity in primary afferent terminals throughout the spinal cord dorsal horn. Eur J Neurosci. 1993;5:1050–1061. - PubMed
- Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. - PubMed
- Cheunsuang O, Morris R. Spinal lamina I neurons that express neurokinin 1 receptors: morphological analysis. Neuroscience. 2000;97:335–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous