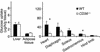Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice - PubMed (original) (raw)
Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice
Tahar Hajri et al. J Clin Invest. 2002 May.
Abstract
Deficiency of the membrane protein FAT/CD36 causes a marked defect in fatty acid uptake by various tissues and is genetically linked to insulin resistance in rats and humans. Here, we examined insulin responsiveness of CD36-/- mice. When fed a diet high in complex carbohydrates and low (5%) in fat, these animals cleared glucose faster than the wild-type. In vivo, uptake of 2-fluorodeoxyglucose by muscle was increased severalfold, and in vitro, insulin responsiveness of glycogenesis by the soleus was enhanced. Null mice had lower glycogen levels in muscle and liver, lower muscle triglyceride levels, and increased liver triglyceride content--all findings consistent with increased insulin-sensitivity. However, when the chow diet was switched to one high in fructose, CD36-/- mice but not wild-type mice developed marked glucose intolerance, hyperinsulinemia, and decreased muscle glucose uptake. High-fat diets impaired glucose tolerance equally in both groups, although CD36 deficiency helped moderate insulin-responsive muscle glucose oxidation. In conclusion, CD36 deficiency enhances insulin responsiveness on a high-starch, low-fat diet. It predisposes to insulin resistance induced by high fructose and partially protects from that induced by high-fat diets. In humans, CD36 deficiency may be an important factor in the metabolic adaptation to diet and in susceptibility to some forms of diet-induced pathology.
Figures
Figure 1
Response of blood glucose (a) and insulin (b) to a glucose load in CD36-null (CD36–/–) and WT mice fed a standard chow diet. Twelve-week-old mice fasted for 16 hours were given glucose (1.5 mg/g) intraperitoneally. (a) Blood glucose was measured before and at 10, 20, 30, 45, 60, 120, and 180 minutes after glucose administration. Two-way repeated-measures ANOVA indicated a significant effect of the genotype (P < 0.05). The change of glucose response over time in each genotype (P < 0.05) and the interaction genotype × glucose are also significant (P < 0.05). *Significant differences (t test) between CD36–/– and WT at each time point. P < 0.01 for 20–60 minutes and P < 0.05 for 120 minutes. Inset shows areas under the glucose tolerance curves (AUC) (P < 0.01). (b) Plasma insulin levels were determined before the glucose injection and at 30 and 60 minutes after injection. *Insulin levels at 0 minutes are significantly lower in CD36–/– than in WT (P < 0.05). All data are means ± SEM with n = 12 (6 males and 6 females).
Figure 2
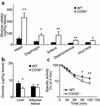
18F-2-FDG uptake (a and b) and FDG blood clearance (c) in CD36-null and WT mice fed the chow diet. Mice were injected with 5 μCi of 18F-2-FDG in a lateral tail vein. Blood samples were collected at 2, 10, 20, 30, 45, 60, 90, and 120 minutes after injection and were tested for radioactivity and glucose content. At the end of the experiment, tissues were removed, weighed, and counted for 18F-2-FDG radioactivity; uptake rate (a and b) is expressed per gram wet tissue. (c) Decay of FDG-specific activity (cpm/μg), calculated as percent of specific activity at 2 minutes after injection, is shown. Data are means ± SEM; n = 6 per group. *P < 0.05, **P < 0.02.
Figure 3
Response of blood glucose (a) and insulin (b) to a glucose load in CD36-null (CD36–/–) and WT mice fed a fructose-rich diet. Mice were fed a diet containing 60% fructose for 12 weeks. After a 16-hour fast, glucose clearance (a) and plasma insulin (b) were tested in response to a glucose load as described in the legend to Figure 1 and in Methods. Data are means ± SEM (n = 7). (a) Two-way repeated-measures ANOVA indicates that change of glucose response over time in each genotype and the interaction genotype × glucose are significant (P < 0.05). *Significant differences (t test) between CD36–/– and WT at each time point. P < 0.01 for 30, 45, and 60 minutes, and P < 0.05 for 20, 90, and 120 minutes. Inset shows areas under the glucose tolerance curves (P < 0.05). (b) *Insulin levels at 0 and 30 minutes are significantly higher in CD36–/– than in WT (P < 0.05).
Figure 4
18F-2-FDG uptake by tissues of CD36-null and WT mice fed a high-fructose diet. 18F-2-FDG (5 μCi) was injected into a lateral tail vein of mice fasted for 16 hours that were maintained on a high-fructose diet for 12 weeks. FDG uptake was determined as described in the legend to Figure 2 and in Methods. Data are means ± SEM (n = 7). *P <0.05.
Figure 5
Response of blood glucose (a) and insulin (b) to a glucose load in CD36-null (CD36–/–) and WT mice fed a high-fat diet. Mice were fed a diet high in safflower oil for 16 weeks. After a 16-hour fast, glucose clearance (a) and plasma insulin (b) were tested in response to a glucose load as described in the legend to Figure 1 and in Methods. Data are means ± SEM (n = 7). (a) Two-way repeated-measures ANOVA indicates that the interaction genotype × glucose is not significantly different between WT and CD36-null mice. Inset shows that area under the glucose response curve for safflower-fed mice (right bars) was significantly different (P < 0.05) from that for mice fed chow (left bars). Black bars, WT mice; white bars, CD36-null mice. *P <0.05. (b) Insulin response to the glucose load in WT and CD36–/– mice. *Insulin levels at 30 minutes are significantly higher in CD36–/– than in WT (P < 0.05).
Similar articles
- Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats.
Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Aitman TJ, et al. Nat Genet. 1999 Jan;21(1):76-83. doi: 10.1038/5013. Nat Genet. 1999. PMID: 9916795 - Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues.
Coburn CT, Hajri T, Ibrahimi A, Abumrad NA. Coburn CT, et al. J Mol Neurosci. 2001 Apr-Jun;16(2-3):117-21; discussion 151-7. doi: 10.1385/JMN:16:2-3:117. J Mol Neurosci. 2001. PMID: 11478366 Review. - A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism.
Bonen A, Han XX, Habets DD, Febbraio M, Glatz JF, Luiken JJ. Bonen A, et al. Am J Physiol Endocrinol Metab. 2007 Jun;292(6):E1740-9. doi: 10.1152/ajpendo.00579.2006. Epub 2007 Jan 30. Am J Physiol Endocrinol Metab. 2007. PMID: 17264223 - [Deletion of CD36 gene ameliorates glucose metabolism abnormality induced by high-fat diet and promotes liver lipid accumulation].
Luo XQ, Zeng H, Tan W, Yang P, Chen YX, Ruan XZ. Luo XQ, et al. Sheng Li Xue Bao. 2021 Oct 25;73(5):805-812. Sheng Li Xue Bao. 2021. PMID: 34708237 Chinese. - New insights into long-chain fatty acid uptake by heart muscle: a crucial role for fatty acid translocase/CD36.
Brinkmann JF, Abumrad NA, Ibrahimi A, van der Vusse GJ, Glatz JF. Brinkmann JF, et al. Biochem J. 2002 Nov 1;367(Pt 3):561-70. doi: 10.1042/BJ20020747. Biochem J. 2002. PMID: 12088505 Free PMC article. Review.
Cited by
- Epigenetic Regulation of Peroxisome Proliferator-Activated Receptor Gamma Mediates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease.
Hajri T, Zaiou M, Fungwe TV, Ouguerram K, Besong S. Hajri T, et al. Cells. 2021 May 31;10(6):1355. doi: 10.3390/cells10061355. Cells. 2021. PMID: 34072832 Free PMC article. - Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice.
Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata L, Thomas A, Pfluger PT, Basford JE, Gilham D, Herz J, Tschöp MH, Hui DY. Hofmann SM, et al. J Clin Invest. 2007 Nov;117(11):3271-82. doi: 10.1172/JCI31929. J Clin Invest. 2007. PMID: 17948131 Free PMC article. - TNAP: A New Multitask Enzyme in Energy Metabolism.
Briolay A, Bessueille L, Magne D. Briolay A, et al. Int J Mol Sci. 2021 Sep 28;22(19):10470. doi: 10.3390/ijms221910470. Int J Mol Sci. 2021. PMID: 34638808 Free PMC article. Review. - Glucose is preferentially utilized for biomass synthesis in pressure-overloaded hearts: evidence from fatty acid-binding protein-4 and -5 knockout mice.
Umbarawan Y, Syamsunarno MRAA, Koitabashi N, Yamaguchi A, Hanaoka H, Hishiki T, Nagahata-Naito Y, Obinata H, Sano M, Sunaga H, Matsui H, Tsushima Y, Suematsu M, Kurabayashi M, Iso T. Umbarawan Y, et al. Cardiovasc Res. 2018 Jul 1;114(8):1132-1144. doi: 10.1093/cvr/cvy063. Cardiovasc Res. 2018. PMID: 29554241 Free PMC article. - Role of adiponectin in the metabolic effects of cannabinoid type 1 receptor blockade in mice with diet-induced obesity.
Tam J, Godlewski G, Earley BJ, Zhou L, Jourdan T, Szanda G, Cinar R, Kunos G. Tam J, et al. Am J Physiol Endocrinol Metab. 2014 Feb 15;306(4):E457-68. doi: 10.1152/ajpendo.00489.2013. Epub 2013 Dec 31. Am J Physiol Endocrinol Metab. 2014. PMID: 24381003 Free PMC article.
References
- Unger, R.H., and Foster, D.W. 1998. Diabetes mellitus. In Williams textbook of endocrinology. 9th edition. J.D. Wilson, D.W. Foster, H.M. Kronenberg, and P.R. Larson, editors. W.B. Saunders Inc. Philadelphia, Pennsylvania, USA. 973–1059.
- Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. - PubMed
- Pan DA, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. - PubMed
- Krssak M, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases