Envelope-dependent, cyclophilin-independent effects of glycosaminoglycans on human immunodeficiency virus type 1 attachment and infection - PubMed (original) (raw)
Envelope-dependent, cyclophilin-independent effects of glycosaminoglycans on human immunodeficiency virus type 1 attachment and infection
Yi-Jun Zhang et al. J Virol. 2002 Jun.
Abstract
Cell surface glycosaminoglycans (GAGs), in particular heparan sulfate (HS), have been proposed to mediate the attachment of human immunodeficiency virus type 1 (HIV-1) to target cells prior to virus entry, and both the viral gp120 envelope protein and virion-associated cyclophilin A (CypA) have been shown to directly interact with HS and its analogues. To determine the role of GAGs in HIV attachment and infection, we generated HIV-susceptible derivatives of CHO cell lines that either express high levels of GAGs (CHO-K1) or lack GAGs (pgsA745). Using a panel of HIV-1 envelopes, we found that cell surface GAG-mediated effects on virion attachment and infection vary in an envelope strain-dependent but coreceptor-independent manner. In fact, cell surface GAG-mediated enhancement of infection is confined to isolates that contain a highly positively charged V3-loop sequence, while infection by most strains is apparently inhibited by the presence of GAGs. Moreover, the enhancing and inhibitory effects of polycations and polyanions on HIV-1 infection are largely dependent on the presence of cell surface GAGs. These observations are consistent with a model in which GAGs influence in vitro HIV-1 infection primarily by modifying the charge characteristics of the target cell surface. Finally, the effects of GAGs on HIV-1 infection are observed to an equivalent extent whether CypA is present in or absent from virions. Overall, these data exclude a major role for GAGs in mediating the attachment of many HIV-1 strains to target cells via interactions with virion-associated gp120 or CypA.
Figures
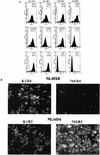
FIG. 1.
HIV-1-susceptible CHO-K1- and pgsA745-derived cell lines. CHO-K1 and pgsA745 cells were sequentially transduced with retroviral vectors encoding mCycT1(Y261C), human CD4, and either human CXCR4 or human CCR5. Cell populations that express approximately equivalent levels of receptors were sorted by FACS. (A) FACS analysis of receptor and HS expression on CHO-K1 (K1/X4 and K1/R5)- and pgsA745 (745/X4 and 745/R5)-derived cell lines. Receptor and HS expression (filled histograms) was measured using allophycocyanin-conjugated anti-CD4 and phycoerythrin-conjugated anti-CXCR4 or anti-CCR5 antibodies. Alternatively, HS expression was measured using an anti-HS IgM monoclonal antibody followed by fluorescein isothiocyanate-conjugated anti-IgM. The log of the mean fluorescent intensity for each cell line is in parentheses. Background staining with isotype control reagents is shown by the open histograms. (B) The same cell lines were inoculated with NL4-3-derived virus stocks. NL/HXB was used for K1/X4 and 745/X4, and NL/ADA was used for K1/R5 and 745/R5. Forty-eight hours postinoculation, infected cells were visualized by immunofluorescent staining using an anti-p24 monoclonal antibody.
FIG. 2.
Variable, envelope-dependent effects of cell surface GAGs on HIV-1 and SIV infectivity. The CHO-K1- and pgsA745-derived target cells described for Fig. 1 that express either the CCR5 or CXCR4 coreceptor were inoculated with serial dilutions of NL4-3-derived viruses bearing R5 (A), X4 (B), or dually tropic (C) envelope proteins. Infection was quantitated 48 h after inoculation in wells that contained an appropriate number of foci (20 to 100) and expressed as focus-forming units (FFU) per microliter of virus-containing supernatant. (D) K1/R5 and 745/R5 target cells were each inoculated with the SIV/GFP reporter virus and the percent GFP-positive, infected cells was determined by FACS 48 h later. The means and standard deviations of three independent infection experiments are shown.
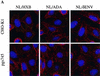
FIG. 3.
Effects of cell surface GAGs on receptor-independent HIV-1 particle attachment. Fluorescent, GFP-Vpr-labeled virions (20 ng of p24) bearing either HXB, ADA, or no envelope proteins were incubated with CHO-K1 or pgsA745 cells on coverslips at 4°C. After 1 h, the cells were washed, fixed, and stained with rhodamine-conjugated concanavalin A and Hoechst 33258 to reveal cellular architecture. (A) The images are a composite of those collected through the entire thickness of the cell monolayer and projected onto a two-dimensional plane. (B) The number of green fluorescent particles attached to at least four individual cells was counted; the means and standard deviations of these counts are shown.
FIG. 3.
Effects of cell surface GAGs on receptor-independent HIV-1 particle attachment. Fluorescent, GFP-Vpr-labeled virions (20 ng of p24) bearing either HXB, ADA, or no envelope proteins were incubated with CHO-K1 or pgsA745 cells on coverslips at 4°C. After 1 h, the cells were washed, fixed, and stained with rhodamine-conjugated concanavalin A and Hoechst 33258 to reveal cellular architecture. (A) The images are a composite of those collected through the entire thickness of the cell monolayer and projected onto a two-dimensional plane. (B) The number of green fluorescent particles attached to at least four individual cells was counted; the means and standard deviations of these counts are shown.
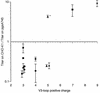
FIG. 4.
Correlation between V3-loop positive charge and GAG effects on HIV-1 infection. For each HIV-1 envelope described for Fig. 2, the overall positive charge of the V3 loop was calculated (some points are slightly offset from the integer value for clarity). The means and standard deviations of the ratio of titers obtained with CHO-K1 and pgsA745 target cells in three experiments are shown. Virus envelopes with distinct coreceptor preferences are depicted by different symbols (circles, R5 strains; triangles, X4 strains; square, dualtropic strain), and a single isolate with an unusually high histidine content within the V3 loop is marked with an asterisk.
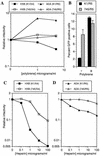
FIG. 5.
Target cell surface GAG-dependent effects of polyanions and polycations on HIV-1 infectivity. (A) CHO-K1- and pgsA745-derived target cells were pretreated with the indicated concentrations of Polybrene prior to infection with R7/HXB/GFP or R7/ADA/GFP. (B) Percent GFP positive K1/R5 and 745/R5 target cells that were untreated or treated with 5 μg of Polybrene per ml prior to infection with R7/ADA/GFP. Alternatively, R7/HXB/GFP (C) and R7/ADA/GFP (D) were incubated with the indicated concentrations of heparin prior to infection of CHO-K1- and pgsA745-derived target cells. For each experiment, the number of infected cells was determined by FACS analysis of GFP expression. Relative infectivity values are numbers of infected cells relative to that obtained with untreated cells and viruses.
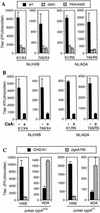
FIG. 6.
The effects of cell surface GAGs on HIV-1 infectivity are independent of CypA incorporation. (A) The infectivity of wild-type or capsid mutant derivatives (G89V and P90A/A92E) of NL/HXB and NL/ADA was measured by using CHO-K1- and pgsA745-derived target cells. The infectious titer of each virus on each cell line was measured as described for Fig. 2 with normalization for minor variation in the amount of p24 in each viral supernatant. (B) NL/HXB and NL/ADA virus stocks were produced in 293T cells in the presence or absence of CsA, and infectious titers were measured after normalization, as for panel A, by using CHO-K1- and pgsA745-derived target cells. (C) NL/HXB and NL/ADA were each pseudotyped with the VSV-G envelope protein and introduced into the unmodified Jurkat cell line or a derivative lacking both copies of the cypA gene. Progeny virions were harvested, and the infectivity of each was measured with CHO-K1 and pgsA745 target cells. For each experiment, the results presented are the means and standard deviations of three titrations.
Similar articles
- DC-SIGN and L-SIGN Are Attachment Factors That Promote Infection of Target Cells by Human Metapneumovirus in the Presence or Absence of Cellular Glycosaminoglycans.
Gillespie L, Gerstenberg K, Ana-Sosa-Batiz F, Parsons MS, Farrukee R, Krabbe M, Spann K, Brooks AG, Londrigan SL, Reading PC. Gillespie L, et al. J Virol. 2016 Aug 12;90(17):7848-63. doi: 10.1128/JVI.00537-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334579 Free PMC article. - Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus.
Chertova E, Bess JW Jr, Crise BJ, Sowder II RC, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. Chertova E, et al. J Virol. 2002 Jun;76(11):5315-25. doi: 10.1128/jvi.76.11.5315-5325.2002. J Virol. 2002. PMID: 11991960 Free PMC article. - Interaction between respiratory syncytial virus and glycosaminoglycans, including heparan sulfate.
Hallak LK, Kwilas SA, Peeples ME. Hallak LK, et al. Methods Mol Biol. 2007;379:15-34. doi: 10.1007/978-1-59745-393-6_2. Methods Mol Biol. 2007. PMID: 17502668 Review. - Glycosaminoglycans and infection.
Aquino RS, Park PW. Aquino RS, et al. Front Biosci (Landmark Ed). 2016 Jun 1;21(6):1260-77. doi: 10.2741/4455. Front Biosci (Landmark Ed). 2016. PMID: 27100505 Free PMC article. Review.
Cited by
- The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription.
Jennings J, Bracey H, Hong J, Nguyen DT, Dasgupta R, Rivera AV, Sluis-Cremer N, Shi J, Aiken C. Jennings J, et al. PLoS Pathog. 2024 Sep 3;20(9):e1011810. doi: 10.1371/journal.ppat.1011810. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39226318 Free PMC article. - The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription.
Jennings J, Bracey H, Nguyen DT, Dasgupta R, Rivera AV, Sluis-Cremer N, Shi J, Aiken C. Jennings J, et al. bioRxiv [Preprint]. 2023 Nov 9:2023.11.08.566350. doi: 10.1101/2023.11.08.566350. bioRxiv. 2023. PMID: 37986899 Free PMC article. Updated. Preprint. - The apparent interferon resistance of transmitted HIV-1 is possibly a consequence of enhanced replicative fitness.
Sugrue E, Wickenhagen A, Mollentze N, Aziz MA, Sreenu VB, Truxa S, Tong L, da Silva Filipe A, Robertson DL, Hughes J, Rihn SJ, Wilson SJ. Sugrue E, et al. PLoS Pathog. 2022 Nov 18;18(11):e1010973. doi: 10.1371/journal.ppat.1010973. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36399512 Free PMC article. - HIV-1 matrix-tRNA complex structure reveals basis for host control of Gag localization.
Bou-Nader C, Muecksch F, Brown JB, Gordon JM, York A, Peng C, Ghirlando R, Summers MF, Bieniasz PD, Zhang J. Bou-Nader C, et al. Cell Host Microbe. 2021 Sep 8;29(9):1421-1436.e7. doi: 10.1016/j.chom.2021.07.006. Epub 2021 Aug 11. Cell Host Microbe. 2021. PMID: 34384537 Free PMC article. - SARS-CoV-2 can recruit a heme metabolite to evade antibody immunity.
Rosa A, Pye VE, Graham C, Muir L, Seow J, Ng KW, Cook NJ, Rees-Spear C, Parker E, Dos Santos MS, Rosadas C, Susana A, Rhys H, Nans A, Masino L, Roustan C, Christodoulou E, Ulferts R, Wrobel AG, Short CE, Fertleman M, Sanders RW, Heaney J, Spyer M, Kjær S, Riddell A, Malim MH, Beale R, MacRae JI, Taylor GP, Nastouli E, van Gils MJ, Rosenthal PB, Pizzato M, McClure MO, Tedder RS, Kassiotis G, McCoy LE, Doores KJ, Cherepanov P. Rosa A, et al. Sci Adv. 2021 May 28;7(22):eabg7607. doi: 10.1126/sciadv.abg7607. Print 2021 May. Sci Adv. 2021. PMID: 33888467 Free PMC article.
References
- Baba, M., R. Snoeck, R. Pauwels, and E. de Clercq. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742-1745. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI36199/AI/NIAID NIH HHS/United States
- R01 AI036199/AI/NIAID NIH HHS/United States
- AI50111/AI/NIAID NIH HHS/United States
- P30 AI042848/AI/NIAID NIH HHS/United States
- R01 AI050111/AI/NIAID NIH HHS/United States
- P30 AI42848/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources