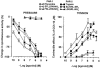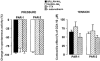Dual effect mediated by protease-activated receptors on the mechanical activity of rat colon - PubMed (original) (raw)
Dual effect mediated by protease-activated receptors on the mechanical activity of rat colon
Flavia Mulè et al. Br J Pharmacol. 2002 Jun.
Abstract
1. The present study examined the mechanical effects of agonist enzymes and receptor-activating peptides for protease-activated receptor (PAR)-1 and PAR-2 on longitudinal and circular muscle of rat isolated colonic segments in the attempt to clarify the PAR functional role in intestinal motility. 2. The responses to PAR-1 and PAR-2 activation were examined in vitro by recording simultaneously the changes of endoluminal pressure (index of circular muscle activity) and of isometric tension (index of longitudinal muscle activity). 3. Both PAR-1 agonists, thrombin (0.1 nM - 3 microM) and SFLLRN-NH2 (1 nM - 3 microM), and PAR-2 agonists, trypsin (0.1 nM - 10 microM) and SLIGRL-NH2 (1 nM - 10 microM), induced different effects in the two muscular layers: a reduction of the spontaneous contractions in the circular muscle and a contractile effect or biphasic, relaxation followed by contraction, depending on the concentration, in the longitudinal muscle. 4. The inhibitory effects were greatly reduced or abolished by apamin (0.1 microM) indicating that they mainly occur via activation of Ca2+-dependent small conductance, K+-channels. 5. The responses to PAR-1 and PAR-2 were unaffected by tetrodotoxin (1 microM) or indomethacin (1 microM) suggesting that are independent by products of cyclooxygenase or by neural action potentials. 6. These findings indicate that both PAR-1 and PAR-2 are functionally expressed in rat colon. PARs mediate changes of the mechanical activity of longitudinal and circular muscle which might explain the alterations of colonic motility observed during inflammatory conditions.
Figures
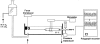
Figure 1
Experimental set-up used for the simultaneous recording of the intraluminal pressure and the isometric tension of the rat intestinal segments.
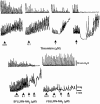
Figure 2
Representative recordings of the effects induced by thrombin and PAR-1 related peptides on the pressure waves (upper tracings) and on the tension oscillations (lower tracings) of an isolated segment of rat colon. The arrow indicates the application of the agonist. Only the more significant concentrations are shown. The PAR-1 agonist, SFLLRN-NH2, and the PAR-1 inactive control peptide, FSLLRN-NH2, were cumulatively added to the bath.
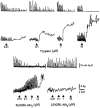
Figure 3
Representative recordings of the effects induced by trypsin and PAR-2 related peptides on the pressure waves (upper tracings) and on the tension oscillations (lower tracings) of an isolated segment of rat colon. The arrow indicates the application of the agonist. Only the more significant concentrations are shown. The PAR-2 agonist, SLIGRL-NH2, and the PAR-2 inactive control peptide, LSIGRL-NH2, were cumulatively added to the bath.
Figure 4
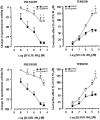
Concentration-response curves for effects evoked by PAR-1 and PAR-2 agonists on the pressure waves (left panel) and on the tension oscillations (right panel) of an isolated segment of rat colon. The inhibitory responses on circular muscle are expressed as the per cent change from the resting spontaneous activity (−100% corresponds to the abolition of spontaneous activity). The contractile effects on longitudinal muscle are expressed as a percentage of the maximal response to carbachol (10 μ
M
). Each value is the mean±s.e.mean of 5 – 9 experiments.
Figure 5
Concentration-response curves to PAR-1 and PAR-2 activating peptides, SFLLRN-NH2 and SLIGRL-NH2, respectively, in rat colonic segments in the absence or in the presence of apamin (0.1 μ
M
). The suppression of spontaneous contractions of circular muscle (left panel) is expressed as the percent change from the resting spontaneous activity (−100% corresponds to the abolition of spontaneous activity). The contractile response on longitudinal muscle (right panel) is expressed as a percentage of the contraction to carbachol (10 μ
M
). Each value is the mean±s.e.mean of six experiments. Apamin significantly antagonized the inhibitory responses and significantly enhanced the contractile effects induced by the highest concentration of PAR-1 agonist. *P<0.05, compared to the control value.
Figure 6
Histograms showing the suppression of spontaneous contractions of circular muscle (left panel) and the contractile effects on longitudinal muscle (right panel) exerted by the PAR-1 agonist, SFLLRN-NH2 (10 μ
M
), or by the PAR-2 agonist, SLIGRL-NH2 (10 μ
M
), in the absence or in the presence of TTX (1 μ
M
) or indomethacin (1 μ
M
). The inhibitory responses were expressed as the percent change from the resting spontaneous activity (−100% corresponds to the abolition of spontaneous activity). The contractile responses are expressed as a percentage of the maximal response to carbachol (10 μ
M
). Each value is the mean±s.e.mean of five experiments. TTX and indomethacin failed to affect the inhibitory and the contractile responses to PAR-1 or PAR-2 activation at all concentrations tested (data not shown).
Similar articles
- Signal transduction pathways involved in the mechanical responses to protease-activated receptors in rat colon.
Mulè F, Baffi MC, Falzone M, Cerra MC. Mulè F, et al. J Pharmacol Exp Ther. 2002 Dec;303(3):1265-72. doi: 10.1124/jpet.102.041301. J Pharmacol Exp Ther. 2002. PMID: 12438551 - Modulation by protease-activated receptors of the rat duodenal motility in vitro: possible mechanisms underlying the evoked contraction and relaxation.
Kawabata A, Kuroda R, Nishikawa H, Kawai K. Kawabata A, et al. Br J Pharmacol. 1999 Oct;128(4):865-72. doi: 10.1038/sj.bjp.0702755. Br J Pharmacol. 1999. PMID: 10556920 Free PMC article. - Dual modulation by thrombin of the motility of rat oesophageal muscularis mucosae via two distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed to PAR-1.
Kawabata A, Kuroda R, Kuroki N, Nishikawa H, Kawai K. Kawabata A, et al. Br J Pharmacol. 2000 Oct;131(3):578-84. doi: 10.1038/sj.bjp.0703590. Br J Pharmacol. 2000. PMID: 11015310 Free PMC article. - Review article: proteinase-activated receptors - novel signals for gastrointestinal pathophysiology.
Vergnolle N. Vergnolle N. Aliment Pharmacol Ther. 2000 Mar;14(3):257-66. doi: 10.1046/j.1365-2036.2000.00690.x. Aliment Pharmacol Ther. 2000. PMID: 10735917 Review. - [Physiological functions of protease-activated receptor-2].
Kawabata A. Kawabata A. Nihon Yakurigaku Zasshi. 2003 Jun;121(6):411-20. doi: 10.1254/fpj.121.411. Nihon Yakurigaku Zasshi. 2003. PMID: 12835535 Review. Japanese.
Cited by
- Modulation of visceral pain and inflammation by protease-activated receptors.
Vergnolle N. Vergnolle N. Br J Pharmacol. 2004 Apr;141(8):1264-74. doi: 10.1038/sj.bjp.0705750. Epub 2004 Mar 29. Br J Pharmacol. 2004. PMID: 15051630 Free PMC article. Review. - Gastrointestinal roles for proteinase-activated receptors in health and disease.
Kawabata A, Matsunami M, Sekiguchi F. Kawabata A, et al. Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S230-40. doi: 10.1038/sj.bjp.0707491. Epub 2007 Nov 12. Br J Pharmacol. 2008. PMID: 17994114 Free PMC article. Review. - Involvement of nitric oxide and tachykinins in the effects induced by protease-activated receptors in rat colon longitudinal muscle.
Mulè F, Baffi MC, Capparelli A, Pizzuti R. Mulè F, et al. Br J Pharmacol. 2003 Jun;139(3):598-604. doi: 10.1038/sj.bjp.0705273. Br J Pharmacol. 2003. PMID: 12788819 Free PMC article. - PARs in the inflammation-cancer transformation of CRC.
Lv J, Liu J, Chao G, Zhang S. Lv J, et al. Clin Transl Oncol. 2023 May;25(5):1242-1251. doi: 10.1007/s12094-022-03052-x. Epub 2022 Dec 22. Clin Transl Oncol. 2023. PMID: 36547764 Review. - Altered functional responses by PAR1 agonist in murine dextran sodium sulphate-treated colon.
Sung TS, Moon SB, Perrino BA, Sanders KM, Koh SD. Sung TS, et al. Sci Rep. 2022 Oct 6;12(1):16746. doi: 10.1038/s41598-022-21285-2. Sci Rep. 2022. PMID: 36202914 Free PMC article.
References
- AL-ANI B., SAIFEDDINE M., HOLLENBERG M.D. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can. J. Physiol. Pharmacol. 1995;73:1203–1207. - PubMed
- COCKS T.M., MOFFATT J.D. Proteinase-activated receptors: sentries for inflammation. Trends Pharmacol. Sci. 2000;21:103–108. - PubMed
- COCKS T.M., SOZZI V., MOFFATT J.D., SELEMIDIS S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999;116:586–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous