The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation - PubMed (original) (raw)
The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation
Latha Shivakumar et al. Mol Cell Biol. 2002 Jun.
Abstract
The RASSF1A locus at 3p21.3 is epigenetically inactivated at high frequency in a variety of solid tumors. Expression of RASSF1A is sufficient to revert the tumorigenicity of human cancer cell lines. We show here that RASSF1A can induce cell cycle arrest by engaging the Rb family cell cycle checkpoint. RASSF1A inhibits accumulation of native cyclin D1, and the RASSF1A-induced cell cycle arrest can be relieved by ectopic expression of cyclin D1 or of other downstream activators of the G(1)/S-phase transition (cyclin A and E7). Regulation of cyclin D1 is responsive to native RASSF1A activity, because RNA interference-mediated downregulation of endogenous RASSF1A expression in human epithelial cells results in abnormal accumulation of cyclin D1 protein. Inhibition of cyclin D1 by RASSF1A occurs posttranscriptionally and is likely at the level of translational control. Rare alleles of RASSF1A, isolated from tumor cell lines, encode proteins that fail to block cyclin D1 accumulation and cell cycle progression. These results strongly suggest that RASSF1A is an important human tumor suppressor protein acting at the level of G(1)/S-phase cell cycle progression.
Figures
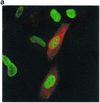
FIG. 1.
RASSF1A blocks proliferation but does not induce apoptosis in human lung carcinoma cells. (a) H1299 cells were transiently transfected with Myc-tagged RASSF1A and placed in suspension cultures 24 h later. After 48 h of incubation in suspension cultures, cells were spun onto glass coverslips, fixed, stained with anti-Myc antibody to detect RASSF1A expression, and labeled by TUNEL to detect fragmented DNA. Cells were treated with 1 μM staurosporine for 4 h in suspension as a positive control for TUNEL (ST/TUNEL). (b) Twenty-four hours after transfection with the indicated constructs, H1299 cells were incubated for an additional 24 h in the presence of BrdU. RASSF1A expression was detected as in panel A. BrdU incorporation was detected with anti-BrdU antibody. An overlay image is shown. Quantitation by microscopic observation of three independent experiments is shown in Table 1. (c) Asynchronous H1299 cells were transfected with GFP or myc-RASSF1A. Forty-eight hours posttransfection, cells were trypsinized, fixed, and stained with propidium iodide. RASSF1A-transfected cultures were additionally stained with anti-Myc and fluorescein-conjugated anti-mouse secondary antibodies. Two-color FACS was used to determine the DNA content of GFP- or RASSF1A-expressing cells. Over 2,000 cells are scored for each analysis. The results shown are representative of three independent experiments. Quantitation of the population of cells in the indicated peaks is shown as a percentage of total events from the three experiments.
FIG. 1.
RASSF1A blocks proliferation but does not induce apoptosis in human lung carcinoma cells. (a) H1299 cells were transiently transfected with Myc-tagged RASSF1A and placed in suspension cultures 24 h later. After 48 h of incubation in suspension cultures, cells were spun onto glass coverslips, fixed, stained with anti-Myc antibody to detect RASSF1A expression, and labeled by TUNEL to detect fragmented DNA. Cells were treated with 1 μM staurosporine for 4 h in suspension as a positive control for TUNEL (ST/TUNEL). (b) Twenty-four hours after transfection with the indicated constructs, H1299 cells were incubated for an additional 24 h in the presence of BrdU. RASSF1A expression was detected as in panel A. BrdU incorporation was detected with anti-BrdU antibody. An overlay image is shown. Quantitation by microscopic observation of three independent experiments is shown in Table 1. (c) Asynchronous H1299 cells were transfected with GFP or myc-RASSF1A. Forty-eight hours posttransfection, cells were trypsinized, fixed, and stained with propidium iodide. RASSF1A-transfected cultures were additionally stained with anti-Myc and fluorescein-conjugated anti-mouse secondary antibodies. Two-color FACS was used to determine the DNA content of GFP- or RASSF1A-expressing cells. Over 2,000 cells are scored for each analysis. The results shown are representative of three independent experiments. Quantitation of the population of cells in the indicated peaks is shown as a percentage of total events from the three experiments.
FIG. 1.
RASSF1A blocks proliferation but does not induce apoptosis in human lung carcinoma cells. (a) H1299 cells were transiently transfected with Myc-tagged RASSF1A and placed in suspension cultures 24 h later. After 48 h of incubation in suspension cultures, cells were spun onto glass coverslips, fixed, stained with anti-Myc antibody to detect RASSF1A expression, and labeled by TUNEL to detect fragmented DNA. Cells were treated with 1 μM staurosporine for 4 h in suspension as a positive control for TUNEL (ST/TUNEL). (b) Twenty-four hours after transfection with the indicated constructs, H1299 cells were incubated for an additional 24 h in the presence of BrdU. RASSF1A expression was detected as in panel A. BrdU incorporation was detected with anti-BrdU antibody. An overlay image is shown. Quantitation by microscopic observation of three independent experiments is shown in Table 1. (c) Asynchronous H1299 cells were transfected with GFP or myc-RASSF1A. Forty-eight hours posttransfection, cells were trypsinized, fixed, and stained with propidium iodide. RASSF1A-transfected cultures were additionally stained with anti-Myc and fluorescein-conjugated anti-mouse secondary antibodies. Two-color FACS was used to determine the DNA content of GFP- or RASSF1A-expressing cells. Over 2,000 cells are scored for each analysis. The results shown are representative of three independent experiments. Quantitation of the population of cells in the indicated peaks is shown as a percentage of total events from the three experiments.
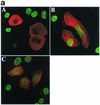
FIG. 2.
RASSF1A variants isolated from tumor cell lines are unable to block proliferation. (a) H1299 cells were transfected with RASSF1A (A), RASSF1A(S131F) (B), or RASSF1A(A133S) (C) and processed as in Fig. 1b. Overlay images are shown with rhodamine red X-conjugated anti-rabbit IgG to detect the anti-Myc polyclonal antibody, and FITC-conjugated anti-mouse IgG to detect the anti-BrdU monoclonal antibody. Quantitation by microscopic observation of at least three independent experiments is shown in Table 1. (b) H1299 cells expressing the carboxy-terminal halves of the indicated RASSF1 variants were labeled with 32Pi for 4 h. RASSF1 variants were immunoprecipitated, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and exposed to PhosphorImager plates to detect incorporation of 32Pi (top panels). Total RASSF1 was detected by Western blotting with anti-Myc antibody (bottom panels). w.t., wild type.
FIG. 2.
RASSF1A variants isolated from tumor cell lines are unable to block proliferation. (a) H1299 cells were transfected with RASSF1A (A), RASSF1A(S131F) (B), or RASSF1A(A133S) (C) and processed as in Fig. 1b. Overlay images are shown with rhodamine red X-conjugated anti-rabbit IgG to detect the anti-Myc polyclonal antibody, and FITC-conjugated anti-mouse IgG to detect the anti-BrdU monoclonal antibody. Quantitation by microscopic observation of at least three independent experiments is shown in Table 1. (b) H1299 cells expressing the carboxy-terminal halves of the indicated RASSF1 variants were labeled with 32Pi for 4 h. RASSF1 variants were immunoprecipitated, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and exposed to PhosphorImager plates to detect incorporation of 32Pi (top panels). Total RASSF1 was detected by Western blotting with anti-Myc antibody (bottom panels). w.t., wild type.
FIG.3.
Bypass of the Rb family cell cycle restriction point allows proliferation of RASSF1A-expressing cells. (a) H1299 cells expressing the indicated constructs together with RASSF1A were assayed for expression and BrdU incorporation. The percentage of cells expressing RASSF1A and incorporating BrdU was quantitated by microscopic observation. Bars represent the standard error from the mean of values obtained from three independent experiments. (b) H1299 cells were transiently transfected with RASSF1A together with cyclin A and assayed for expression of RASSF1A (A), cyclin A (B), and BrdU incorporation (C). Panel D is an overlay of images A to C.
FIG.3.
Bypass of the Rb family cell cycle restriction point allows proliferation of RASSF1A-expressing cells. (a) H1299 cells expressing the indicated constructs together with RASSF1A were assayed for expression and BrdU incorporation. The percentage of cells expressing RASSF1A and incorporating BrdU was quantitated by microscopic observation. Bars represent the standard error from the mean of values obtained from three independent experiments. (b) H1299 cells were transiently transfected with RASSF1A together with cyclin A and assayed for expression of RASSF1A (A), cyclin A (B), and BrdU incorporation (C). Panel D is an overlay of images A to C.
FIG. 4.
Ras12V expression does not bypass RASSF1A-mediated growth arrest. HME50-hTERT cells were transfected with the indicated constructs and processed as described in the legend to Fig. 3b. The percentage of RASSF1A-expressing cells and RASSF1A+Ras12V-expressing cells incorporating BrdU is shown normalized to the BrdU incorporation frequency of cells expressing Ras12V alone (approximately 80% of total cells).
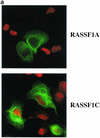
FIG. 5.
RASSF1A prevents accumulation of cyclin D1 protein. (a) H1299 cells were transfected with RASSF1A or RASSF1C and stained with anti-cyclin D1 and anti-Myc antibodies. Overlays are shown with rhodamine red X-conjugated anti-rabbit IgG antibodies to detect rabbit anti-cyclin D and FITC-conjugated anti-mouse IgG to detect the 9E10 anti-Myc antibody. Quantitation by microscopic observation of at least three independent experiments is shown in Table1. (b) H1299 cells or HME cells were transfected with the indicated constructs together with a luciferase reporter construct driven by the human cyclin D1 promoter (−1745 CD1-Luc). Relative luciferase values are shown normalized to activities obtained with empty vector (H1299) or Ras12V alone (HME). Ras12V expression resulted in fourfold induction of luciferase activity in HME cells over baseline levels observed in serum-starved cells. Bars indicate the standard error from the mean of average values from three independent experiments performed in duplicate. (c) MEFs derived from wild-type and cyclin D1−/− mice were transiently transfected with the indicated constructs and assayed for BrdU incorporation as described in the legend to Fig. 1.
FIG. 5.
RASSF1A prevents accumulation of cyclin D1 protein. (a) H1299 cells were transfected with RASSF1A or RASSF1C and stained with anti-cyclin D1 and anti-Myc antibodies. Overlays are shown with rhodamine red X-conjugated anti-rabbit IgG antibodies to detect rabbit anti-cyclin D and FITC-conjugated anti-mouse IgG to detect the 9E10 anti-Myc antibody. Quantitation by microscopic observation of at least three independent experiments is shown in Table1. (b) H1299 cells or HME cells were transfected with the indicated constructs together with a luciferase reporter construct driven by the human cyclin D1 promoter (−1745 CD1-Luc). Relative luciferase values are shown normalized to activities obtained with empty vector (H1299) or Ras12V alone (HME). Ras12V expression resulted in fourfold induction of luciferase activity in HME cells over baseline levels observed in serum-starved cells. Bars indicate the standard error from the mean of average values from three independent experiments performed in duplicate. (c) MEFs derived from wild-type and cyclin D1−/− mice were transiently transfected with the indicated constructs and assayed for BrdU incorporation as described in the legend to Fig. 1.
FIG. 5.
RASSF1A prevents accumulation of cyclin D1 protein. (a) H1299 cells were transfected with RASSF1A or RASSF1C and stained with anti-cyclin D1 and anti-Myc antibodies. Overlays are shown with rhodamine red X-conjugated anti-rabbit IgG antibodies to detect rabbit anti-cyclin D and FITC-conjugated anti-mouse IgG to detect the 9E10 anti-Myc antibody. Quantitation by microscopic observation of at least three independent experiments is shown in Table1. (b) H1299 cells or HME cells were transfected with the indicated constructs together with a luciferase reporter construct driven by the human cyclin D1 promoter (−1745 CD1-Luc). Relative luciferase values are shown normalized to activities obtained with empty vector (H1299) or Ras12V alone (HME). Ras12V expression resulted in fourfold induction of luciferase activity in HME cells over baseline levels observed in serum-starved cells. Bars indicate the standard error from the mean of average values from three independent experiments performed in duplicate. (c) MEFs derived from wild-type and cyclin D1−/− mice were transiently transfected with the indicated constructs and assayed for BrdU incorporation as described in the legend to Fig. 1.
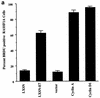
FIG. 6.
Enhanced accumulation of cylin D1 in the absence of RASSF1A expression. (a) BrdU incorporation in RASSF1A-expressing HeLa cells was assayed as described in Fig. 1. (b) HeLa cells were exposed to siRNA specifically targeting the RASSF1A transcript for 72 h. siRNA directed against caveolin 1 was used as a negative control. Cyclin D1 levels in cells exposed to caveolin 1 siRNA are identical to those in untreated cells (data not shown). Whole-cell lysates were assayed for expression of the indicated proteins. Ten micrograms of total protein was loaded for each sample. (c) RPAs were performed to examine the relative amounts of cyclin D1 mRNA present in cells treated as described for panel b.
FIG. 6.
Enhanced accumulation of cylin D1 in the absence of RASSF1A expression. (a) BrdU incorporation in RASSF1A-expressing HeLa cells was assayed as described in Fig. 1. (b) HeLa cells were exposed to siRNA specifically targeting the RASSF1A transcript for 72 h. siRNA directed against caveolin 1 was used as a negative control. Cyclin D1 levels in cells exposed to caveolin 1 siRNA are identical to those in untreated cells (data not shown). Whole-cell lysates were assayed for expression of the indicated proteins. Ten micrograms of total protein was loaded for each sample. (c) RPAs were performed to examine the relative amounts of cyclin D1 mRNA present in cells treated as described for panel b.
FIG. 6.
Enhanced accumulation of cylin D1 in the absence of RASSF1A expression. (a) BrdU incorporation in RASSF1A-expressing HeLa cells was assayed as described in Fig. 1. (b) HeLa cells were exposed to siRNA specifically targeting the RASSF1A transcript for 72 h. siRNA directed against caveolin 1 was used as a negative control. Cyclin D1 levels in cells exposed to caveolin 1 siRNA are identical to those in untreated cells (data not shown). Whole-cell lysates were assayed for expression of the indicated proteins. Ten micrograms of total protein was loaded for each sample. (c) RPAs were performed to examine the relative amounts of cyclin D1 mRNA present in cells treated as described for panel b.
Similar articles
- Identification of novel gene expression targets for the Ras association domain family 1 (RASSF1A) tumor suppressor gene in non-small cell lung cancer and neuroblastoma.
Agathanggelou A, Bièche I, Ahmed-Choudhury J, Nicke B, Dammann R, Baksh S, Gao B, Minna JD, Downward J, Maher ER, Latif F. Agathanggelou A, et al. Cancer Res. 2003 Sep 1;63(17):5344-51. Cancer Res. 2003. PMID: 14500366 Free PMC article. - [Inhibitory effect of wild-type RASSF1A gene expression on proliferation of hepatocellular carcinoma QGY-7703 cells].
Rui L, Xue WJ, Li P, Wang ZW, Wang P, Li HX. Rui L, et al. Ai Zheng. 2008 Sep;27(9):924-8. Ai Zheng. 2008. PMID: 18799029 Chinese. - Differential requirements for ras and the retinoblastoma tumor suppressor protein in the androgen dependence of prostatic adenocarcinoma cells.
Fribourg AF, Knudsen KE, Strobeck MW, Lindhorst CM, Knudsen ES. Fribourg AF, et al. Cell Growth Differ. 2000 Jul;11(7):361-72. Cell Growth Differ. 2000. PMID: 10939590 - [Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].
Viallard JF, Lacombe F, Belloc F, Pellegrin JL, Reiffers J. Viallard JF, et al. Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French. - Cycling to cancer with cyclin D1.
Diehl JA. Diehl JA. Cancer Biol Ther. 2002 May-Jun;1(3):226-31. doi: 10.4161/cbt.72. Cancer Biol Ther. 2002. PMID: 12432268 Review.
Cited by
- Extracellular Superoxide Dismutase (EC-SOD) Regulates Gene Methylation and Cardiac Fibrosis During Chronic Hypoxic Stress.
Rajgarhia A, Ayasolla KR, Zaghloul N, Lopez Da Re JM, Miller EJ, Ahmed M. Rajgarhia A, et al. Front Cardiovasc Med. 2021 May 31;8:669975. doi: 10.3389/fcvm.2021.669975. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34136546 Free PMC article. - CRISPR-Cas9-mediated gene therapy in lung cancer.
Kazemizadeh H, Kashefizadeh A. Kazemizadeh H, et al. Clin Transl Oncol. 2023 May;25(5):1156-1166. doi: 10.1007/s12094-022-03039-8. Epub 2022 Dec 10. Clin Transl Oncol. 2023. PMID: 36495467 Review. - RASSF1 Polymorphisms in Cancer.
Gordon M, El-Kalla M, Baksh S. Gordon M, et al. Mol Biol Int. 2012;2012:365213. doi: 10.1155/2012/365213. Epub 2012 May 31. Mol Biol Int. 2012. PMID: 22701175 Free PMC article. - Release of RASSF1C from the nucleus by Daxx degradation links DNA damage and SAPK/JNK activation.
Kitagawa D, Kajiho H, Negishi T, Ura S, Watanabe T, Wada T, Ichijo H, Katada T, Nishina H. Kitagawa D, et al. EMBO J. 2006 Jul 26;25(14):3286-97. doi: 10.1038/sj.emboj.7601212. Epub 2006 Jun 29. EMBO J. 2006. PMID: 16810318 Free PMC article. - EBNA3C facilitates RASSF1A downregulation through ubiquitin-mediated degradation and promoter hypermethylation to drive B-cell proliferation.
Zhang S, Pei Y, Lang F, Sun K, Singh RK, Lamplugh ZL, Saha A, Robertson ES. Zhang S, et al. PLoS Pathog. 2019 Jan 7;15(1):e1007514. doi: 10.1371/journal.ppat.1007514. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30615685 Free PMC article.
References
- Agami, R., and R. Bernards. 2000. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102:55-66. - PubMed
- Albanese, C., M. D'Amico, A. T. Reutens, M. Fu, G. Watanabe, R. J. Lee, R. N. Kitsis, B. Henglein, M. Avantaggiati, K. Somasundaram, B. Thimmapaya, and R. G. Pestell. 1999. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274:34186-34195. - PubMed
- Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R. G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589-23597. - PubMed
- Assoian, R. K., and M. A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48-53. - PubMed
- Burbee, D. G., E. Forgacs, S. Zochbauer-Muller, L. Shivakumar, K. Fong, B. Gao, D. Randle, A. Virmani, S. Bader, Y. Sekido, F. Latif, S. Milchgrub, A. F. Gazdar, M. I. Lerman, M. A. White, and J. D. Minna. 2001. The RASSF1A locus in the 3p21.3 homozygous deletion region: epigenetic inactivation in lung and breast cancer and suppression of the malignant phenotype. Cancer Res. 93:691-699. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA71443/CA/NCI NIH HHS/United States
- R01 CA071618/CA/NCI NIH HHS/United States
- R01CA71618/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- R01 CA071443/CA/NCI NIH HHS/United States
- R01CA70896/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous