Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice - PubMed (original) (raw)
. 2002 Jun 3;21(11):2591-601.
doi: 10.1093/emboj/21.11.2591.
Yunhua Chang, Martine Manuel, Tero-Pekka Alastalo, Murielle Rallu, Yorick Gitton, Lila Pirkkala, Marie-Thérèse Loones, Liliana Paslaru, Severine Larney, Sophie Hiard, Michel Morange, Lea Sistonen, Valérie Mezger
Affiliations
- PMID: 12032072
- PMCID: PMC125382
- DOI: 10.1093/emboj/21.11.2591
Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice
Marko Kallio et al. EMBO J. 2002.
Abstract
Heat shock factor 2, one of the four vertebrate HSFs, transcriptional regulators of heat shock gene expression, is active during embryogenesis and spermatogenesis, with unknown functions and targets. By disrupting the Hsf2 gene, we show that, although the lack of HSF2 is not embryonic lethal, Hsf2(-/-) mice suffer from brain abnormalities, and meiotic and gameto genesis defects in both genders. The disturbances in brain are characterized by the enlargement of lateral and third ventricles and the reduction of hippocampus and striatum, in correlation with HSF2 expression in proliferative cells of the neuroepithelium and in some ependymal cells in adults. Many developing spermatocytes are eliminated via apoptosis in a stage-specific manner in Hsf2(-/-) males, and pachytene spermatocytes also display structural defects in the synaptonemal complexes between homologous chromosomes. Hsf2(-/-) females suffer from multiple fertility defects: the production of abnormal eggs, the reduction in ovarian follicle number and the presence of hemorrhagic cystic follicles are consistent with meiotic defects. Hsf2(-/-) females also display hormone response defects, that can be rescued by superovulation treatment, and exhibit abnormal rates of luteinizing hormone receptor mRNAs.
Figures
Fig. 1. Targeted inactivation of the Hsf2 gene. (A) Schematic representation of the wild-type and mutated alleles. Horizontal small arrows show the location of the three primers used for PCR genotyping. (B) PCR genotyping of offspring from F1 heterozygous intercrosses. (C) Southern blot of _Bam_HI-digested tail DNA. Lanes 1 and 3: wild-type allele 6.5 kb fragment. Lanes 2 and 3: disrupted allele 4 kb fragment. (D) RT–PCR analysis of HSF2, chimeric HSF2-βgeo, actin and GAPDH mRNA levels in Hsf2+/+, Hsf2+/– and _Hsf2_–/– tissues. Lane 1: no reverse transcription (0); lane 2, molecular markers (MM); lanes 3 and 4, testis; lanes 5 and 6, E9.5 embryos; lanes 7 and 8, E13.5 embryos. (E) Western blot analysis of whole E11.5 embryo extracts of littermates with polyclonal anti-mouse HSF2 (upper panel). Equal loading and transfer were assessed with a monoclonal anti-HSF1 (lower panel). Lanes 1–3 and 11–13, Hsf2+/+; lanes 4–6 and 14–17, _Hsf2_–/–; lanes 7–9 and 17, Hsf2+/–; lane 10, unstressed F9 EC cells. (F) EMSA analysis of E11.5 embryo extracts with an HSE-containing double-stranded oligonucleotide. Lane 1, no extracts; lanes 2 and 6–11, unstressed F9 EC cells; lanes 3–5 and 12–23, embryos. The dilutions of polyclonal anti-mouse HSF2 in supershift experiment are indicated. Arrowheads, specific HSF2–HSE complexes; ns, non-specific complexes.
Fig. 2. LacZ expression as a reporter of the HSF2 expression profile. (A and B) Lateral view of an E9.5 and E13.5 _Hsf2_–/– embryo, respectively. (C) Dorsal view of an E15.5 _Hsf2_–/– embryo. (D) Transverse section of a seminiferous tubule showing X-gal staining of spermatocytes. (E) Cytoplasmic localization of the chimeric recombinant protein using β-galactosidase detection by X-gal staining (blue) in mouse pachytene spermatocytes (_Hsf2_–/– male). nuc, nucleus; h, heart; so, somites; pro, prosencephalon; mes, mesencephalon; met, metencephalon; myel, myel encephalon; tel, telencephalon; ba, branchial arches; fb, forebrain; mb, midbrain; sp, spinal chord; msp, mature spermatozoa; sg, spermatogonia; sc, spermatocytes; st, spermatids.
Fig. 3. HSF2 and β-gal expression in the ventricular zone of embryonic and adult brain. (A) Parasagittal section of an E13.5 _Hsf2_–/– embryonic brain showing β-gal expression along the lumen of the ventricles. fb, forebrain; mb, midbrain; chp, choroid plexus. (B and C) HSF2 immunolocalization and BrdU staining, respectively, at the level of midbrain in E12.5 Hsf2+/+ embryos. vz, ventricular zone; lv, lumen of the ventricle. (D–F) β-gal detection in the ependymal layer of adult _Hsf2_–/– brain. st, striatum; se, septum; ep, ependymal zone; lv, lateral ventricle. Scale bar: 50 µm. (E) Detail of (D), magnification: 20×. (F) Detail of a transverse section at the level of the hippocampus (h), magnification: 20×.
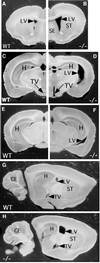
Fig. 4. Abnormal structure of the HSF2-deficient adult brain. Brain transverse sections (A–F) from Hsf2+/+ (A, C and E) or _Hsf2_–/– (B, D and F) 3-month-old males, illustrating the enlargment of the lateral and third ventricles all along the brain. (G and H) Parasagittal sections of the adult brain showing the reduced size of the hippocampus in _Hsf2_–/– (–/–) (H) compared with Hsf2+/+ (WT) (G) brain. ST, striatum; SE, septum; H, hippocampus; LV, lateral ventricle; TV, third ventricle; CE, cerebellum.
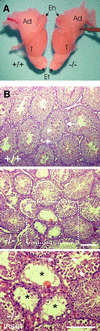
Fig. 5. Gross anatomy of male reproductive organs and analysis of testis cross-sections of adult Hsf2+/+ (+/+) and _Hsf2_–/– mice (–/–). (A) Testis and epididymis size. T, testis; Eh, head of epididymis; Et, tail of epididymis; Ad, adipose tissues. (B) Cross-sections from testes. For (+/+): arrowhead, elongating spermatids; short arrow, spermatocytes; long arrow, spermatogonia. For (–/–): reduction in the diameter of the seminiferous tubules and indications of disruption of spermatogenesis. For inset: lack of spermatocytes (short arrow) and spermatids (arrowhead), vacuolization of the tubules (asterisk). Bars = 100 µm.
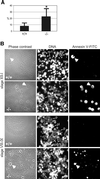
Fig. 6. Apoptosis of developing germ cells in the testes of _Hsf2_–/– mice. (A) Flow cytometric analysis of annexin V–FITC-stained testicular cells. (B) Stage-specific apoptosis in the testis of _Hsf2_–/– mice. Translumination-assisted dissection of the seminiferous tubules followed by annexin V–FITC immunofluorescence and microscopic analysis revealed two populations of dying cells in the _Hsf2_–/– mice at stages VIII–IX and XII–I; late pachytene and meiotically dividing spermatocytes (arrows). Tubule segments isolated from stages XII–I containing type A3 and A4 spermatogonia, early pachytene spermatocytes, meiotically dividing spermatocytes (m1, m2), round step-1 spermatids (rp1) and elongating spermatids (ep), and from stages VIII–IX containing type A1 spermatogonia, pre-leptotene spermatocytes (pl), late pachytene spermatocytes (p) and elongating step-8/9 spermatids (sp8/9). Note the reduction in the number of post-meiotic round and elongated spermatids in the _Hsf2_–/– mice.
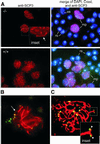
Fig. 7. Synapsis between the homologous chromosomes is defective in the _Hsf2_–/– mice. (A) Immunofluorescence labeling with anti-SCP3 antibody (Cy3 channel, red) and Crest anti-centromere sera (FITC channel, green). A part of a seminiferous tubule in developmental stage VIII (Clermont, 1972), with middle pachytene spermatocytes (mp), round spermatids (rs) and elongated spermatids (es). Loop-like configurations are present between one or more pairs of homologous chromosomes (arrow in the inset A). (B) An SC with one loop-like structure near the centromere terminus of a chromosome pair (arrow) (_Z_-level section). (C) Separation of the lateral elements at the centromere region (arrowheads in the inset C; stack of five _Z_-level sections from a confocal microscope series). Merge of anti-SCP3 (red) and Crest (green) in images (B) and (C). Bars = 10 µm.
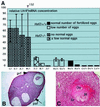
Fig. 8. Ovarian defects in _Hsf2_–/– females. (A) Abnormally elevated levels of luteinizing hormone receptor (LH-R) mRNAs in _Hsf2_–/– with anovulatory problems. Seven Hsf2+/+ and seven _Hsf2_–/– 5-month-old females were mated with OF1 males. On the day of plug detection, eggs were harvested and counted in the ampulla. In parallel, LH-R mRNA levels were analyzed by RT–PCR. The relative mRNA concentration is given in arbitrary units. Value 1 was attributed to the ovary exhibiting the lowest expression level. Each plot corresponds to the average determined from three or four PCR experiments (except for female B that was tested only once). Error bars are indicated. Dense hatched bars, _Hsf2_–/– females that produced no eggs or only abnormal fragmented eggs. Hatched bars, _Hsf2_–/– females that produced a few (an average of two) fertilized eggs. Empty bars, Hsf2+/+ females with no ovulated fertilized eggs. Filled bars, Hsf2+/+ females with normal ovulation scores and a normal number of fertilized eggs. (B) Paraffin section of an _Hsf2_–/– ovary with marked ovulatory problems: low number of primary (prf) and pre-antral (paf) follicles. (C) A pre-antral hemorrhagic follicle in the section of an _Hsf2_–/– ovary which never gave offspring. Oo, oocyte; Gr, granulosa cells; Bl, blood.
Similar articles
- Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility.
Wang G, Ying Z, Jin X, Tu N, Zhang Y, Phillips M, Moskophidis D, Mivechi NF. Wang G, et al. Genesis. 2004 Feb;38(2):66-80. doi: 10.1002/gene.20005. Genesis. 2004. PMID: 14994269 - Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium.
Alastalo TP, Lönnström M, Leppä S, Kaarniranta K, Pelto-Huikko M, Sistonen L, Parvinen M. Alastalo TP, et al. Exp Cell Res. 1998 Apr 10;240(1):16-27. doi: 10.1006/excr.1997.3926. Exp Cell Res. 1998. PMID: 9570917 - The Role of Heat Shock Factors in Mammalian Spermatogenesis.
Widlak W, Vydra N. Widlak W, et al. Adv Anat Embryol Cell Biol. 2017;222:45-65. doi: 10.1007/978-3-319-51409-3_3. Adv Anat Embryol Cell Biol. 2017. PMID: 28389750 Review.
Cited by
- Splice variants and seasonal expression of buffalo HSF genes.
Lal SV, Brahma B, Gohain M, Mohanta D, De BC, Chopra M, Dass G, Vats A, Upadhyay RC, Datta TK, De S. Lal SV, et al. Cell Stress Chaperones. 2015 May;20(3):545-54. doi: 10.1007/s12192-014-0563-y. Epub 2015 Feb 6. Cell Stress Chaperones. 2015. PMID: 25655489 Free PMC article. - Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression.
Chang Y, Ostling P, Akerfelt M, Trouillet D, Rallu M, Gitton Y, El Fatimy R, Fardeau V, Le Crom S, Morange M, Sistonen L, Mezger V. Chang Y, et al. Genes Dev. 2006 Apr 1;20(7):836-47. doi: 10.1101/gad.366906. Genes Dev. 2006. PMID: 16600913 Free PMC article. - New insights into the genetics of spermatogenic failure: a review of the literature.
Cannarella R, Condorelli RA, Duca Y, La Vignera S, Calogero AE. Cannarella R, et al. Hum Genet. 2019 Feb;138(2):125-140. doi: 10.1007/s00439-019-01974-1. Epub 2019 Jan 17. Hum Genet. 2019. PMID: 30656449 Review. - Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli.
Sandqvist A, Björk JK, Akerfelt M, Chitikova Z, Grichine A, Vourc'h C, Jolly C, Salminen TA, Nymalm Y, Sistonen L. Sandqvist A, et al. Mol Biol Cell. 2009 Mar;20(5):1340-7. doi: 10.1091/mbc.e08-08-0864. Epub 2009 Jan 7. Mol Biol Cell. 2009. PMID: 19129477 Free PMC article. - Rare double sex and mab-3-related transcription factor 1 regulatory variants in severe spermatogenic failure.
Lima AC, Carvalho F, Gonçalves J, Fernandes S, Marques PI, Sousa M, Barros A, Seixas S, Amorim A, Conrad DF, Lopes AM. Lima AC, et al. Andrology. 2015 Sep;3(5):825-33. doi: 10.1111/andr.12063. Epub 2015 Jul 2. Andrology. 2015. PMID: 26139570 Free PMC article.
References
- Alastalo T.P., Lonnstrom,M., Leppa,S., Kaarniranta,K., Pelto-Huikko,M., Sistonen,L. and Parvinen,M. (1998) Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium. Exp. Cell Res., 240, 16–27. - PubMed
- Baudat F., Manova,K., Pui Yuen,J., Jasin,M. and Keeney,S. (2000) Chromosone synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell, 6, 989–998. - PubMed
- Christians E., Davis,A.A., Thomas,S.D. and Benjamin,I.J. (2000) Maternal effect of Hsf1 on reproductive success. Nature, 407, 693–694. - PubMed
- Clermont Y. (1972) Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonia renewal. Physiol. Rev., 52, 198–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases