15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis - PubMed (original) (raw)
15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis
Mitsuhiro Kondo et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Prostaglandin D(2) (PGD(2)), a major cyclooxygenase product in a variety of tissues and cells, readily undergoes dehydration to yield the bioactive cyclopentenone-type PGs of the J(2)-series, such as 15-deoxy-Delta(12,14)-PGJ(2) (15d-PGJ(2)). The observation that the level of 15d-PGJ(2) increased in the tissue cells from patients with sporadic amyotrophic lateral sclerosis suggested that the formation of 15d-PGJ(2) may be closely associated with neuronal cell death during chronic inflammatory processes. In vitro experiments using SH-SY5Y human neuroblastoma cells revealed that 15d-PGJ(2) induced apoptotic cell death. An oligonucleotide microarray analysis demonstrated that, in addition to the heat shock-responsive and redox-responsive genes, the p53-responsive genes, such as gadd45, cyclin G1, and cathepsin D, were significantly up-regulated in the cells treated with 15d-PGJ(2). Indeed, the 15d-PGJ(2) induced accumulation and phosphorylation of p53, which was accompanied by a preferential redistribution of the p53 protein in the nuclei of the cells and by a time-dependent increase in p53 DNA binding activity, suggesting that p53 accumulated in response to the treatment with 15d-PGJ(2) was functional. The 15d-PGJ(2)-induced accumulation of p53 resulted in the activation of a death-inducing caspase cascade mediated by Fas and the Fas ligand.
Figures
Figure 1
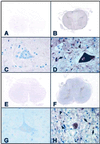
Photomicrographs of spinal cord sections from control cases (A, C, E, and G) and ALS cases (B, D, F, and H) immunostained with antibodies specific for COX-2 (A_–_D) and 15d-PGJ2 (E_–_H). The control spinal cords were negatively stained with the antibodies to COX-2 (A and C) and 15d-PGJ2 (E and G). In the ALS spinal cords, both of these antibodies reacted with the gray matter but not with the white matter (B and F). These immunoreactivities were more intense in the perikarya and proximal cell processes of the anterior horn cells, compared with the reactive astrocytes (arrows), microglia/macrophages (arrowheads), and surrounding neuropil to a lesser extent. (A_–_D) Formalin-fixed, paraffin-embedded sections; (E-H) OCT compound-embedded, frozen sections; (A-H) avidin-biotin-immunoperoxidase complex method. (A, B, E, and F) ×6; (C, D, G, and H) ×40.
Figure 2
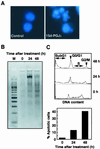
Induction of apoptosis in SH-SY5Y cells treated with 15d-PGJ2. (A) SH-SY5Y human neuroblastoma cells were fixed with paraformaldehyde, stained with Hoechst 33258, and examined by fluorescence microscopy. (Left) Untreated control cells. (Right) Cells treated with 10 μM 15d-PGJ2 for 24 h. (B) DNA fragmentation in SH-SY5Y cells treated with 10 μM 15d-PGJ2. Nucleosomal DNA fragmentation was visualized by agarose gel electrophoresis. M, DNA size markers. (C) SubG1 analysis of SH-SY5Y cells treated with 15d-PGJ2. SH-SY5Y cells treated with 10 μM 15d-PGJ2 were analyzed for DNA content by PI staining by using a flow cytometer (Upper). The subG1 DNA content was used as indicative of apoptotic cells (Lower). Cells with nuclei condensation, DNA fragmentation, and subG1 DNA contents as typical hallmarks of apoptosis revealed that 15d-PGJ2 induced apoptosis.
Figure 3
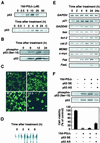
Induction of p53 in SH-SY5Y cells treated with 15d-PGJ2. SH-SY5Y cells were treated for 8 h with 10 μM of the indicated PGs, and p53 induction was examined by immunoblot analysis. (A Upper) Dose-dependent induction of p53 in SH-SY5Y cells treated with 0–50 μM 15d-PGJ2 for 8 h. (Lower) Time-dependent induction of p53 in SH-SY5Y cells treated with 10 μM 15d-PGJ2. (B) Analysis of activated p53 in SH-SY5Y cells treated with 10 μM 15d-PGJ2. (C) Induction of nuclear translocation of p53 in SH-SY5Y cells exposed to 10 μM 15d-PGJ2. The RL34 cells were fixed in 2% paraformaldehyde and 0.2% picric acid and then immunostained with the anti-p53 antibody. Images of the cellular immunofluorescence were acquired by using a confocal laser scanning microscope. (D) 15d-PGJ2 induces nuclear protein-p53 binding activity. SH-SY5Y cells were treated with 10 μM 15d-PGJ2 for different time intervals as indicated in the figures. (E) RT-PCR analysis of p53-responsive gene expression in SH-SY5Y cells treated with 10 μM 15d-PGJ2 for different time intervals. (F) Inhibition of p53 phosphorylation, p53 protein expression, and cell death by p53AS in SH-SY5Y cells treated with 15d-PGJ2. Indicated antisense oligonucleotides (2 μg) were transfected for 12 h in SH-SY5Y cells, and then the cells were treated for 8 h with 10 μM 15d-PGJ2. The induction of p53 was examined by immunoblot analysis, and cell viability was measured by the MTT assay. In the MTT assay, data are expressed as the percentage of control culture conditions.
Figure 4
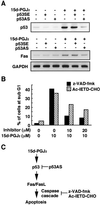
p53-dependent activation of Fas/FasL pathway by 15d-PGJ2. (A) Inhibition of p53 protein expression (Upper) and Fas expression (Lower) by p53AS in SH-SY5Y cells treated with 15d-PGJ2. The p53 sense (p53SE) or antisense (p53AS) oligonucleotides were transfected with Lipofectin (GIBCO) reagents for 12 h in SH-SY5Y cells, and then the cells were treated with 10 μM 15d-PGJ2 for 8 h. Fas induction was examined by RT-PCR analysis. Treatment with Lipofectin alone did not affect the expression of p53 and Fas (data not shown). (B) Effect of caspase inhibitors on 15d-PGJ2-induced increase in number of cells with a subG1 DNA content. The inhibitors used were z-VAD-fmk (hatched bar) and Ac-IETD-CHO (filled bar). The SH-SY5Y cells were treated with 10 μM 15d-PGJ2 in the presence or absence of inhibitor for 8 h. The subG1 DNA contents were analyzed by PI staining by using a flow cytometer. (C) Model for mechanisms by which 15d-PGJ2 exerts p53-mediated neuronal apoptosis.
Similar articles
- An endogenous electrophile that modulates the regulatory mechanism of protein turnover: inhibitory effects of 15-deoxy-Delta 12,14-prostaglandin J2 on proteasome.
Shibata T, Yamada T, Kondo M, Tanahashi N, Tanaka K, Nakamura H, Masutani H, Yodoi J, Uchida K. Shibata T, et al. Biochemistry. 2003 Dec 2;42(47):13960-8. doi: 10.1021/bi035215a. Biochemistry. 2003. PMID: 14636064 - Translational regulation of cyclin D1 by 15-deoxy-delta(12,14)-prostaglandin J(2).
Campo PA, Das S, Hsiang CH, Bui T, Samuel CE, Straus DS. Campo PA, et al. Cell Growth Differ. 2002 Sep;13(9):409-20. Cell Growth Differ. 2002. PMID: 12354750 - 15-Deoxy-Delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses.
Uchida K, Shibata T. Uchida K, et al. Chem Res Toxicol. 2008 Jan;21(1):138-44. doi: 10.1021/tx700177j. Epub 2007 Dec 4. Chem Res Toxicol. 2008. PMID: 18052108 Review. - 15-Deoxy-Delta(12,14)-prostaglandin J(2) induces mitochondrial-dependent apoptosis through inhibition of PKA/NF-kappaB in renal proximal epithelial cells.
Lee DR, Kwon CH, Park JY, Kim YK, Woo JS. Lee DR, et al. Toxicology. 2009 Apr 5;258(1):17-24. doi: 10.1016/j.tox.2009.01.001. Epub 2009 Jan 9. Toxicology. 2009. PMID: 19167456 - Anti- and proinflammatory effects of 15-deoxy-delta-prostaglandin J2(15d-PGJ2) on human eosinophil functions.
Ueki S, Kato H, Kobayashi Y, Ito W, Adachi T, Nagase H, Ohta K, Kayaba H, Chihara J. Ueki S, et al. Int Arch Allergy Immunol. 2007;143 Suppl 1:15-22. doi: 10.1159/000101399. Epub 2007 May 1. Int Arch Allergy Immunol. 2007. PMID: 17541271 Review.
Cited by
- A systematic review for the development of Alzheimer's disease in in vitro models: a focus on different inducing agents.
Prajapat M, Kaur G, Choudhary G, Pahwa P, Bansal S, Joshi R, Batra G, Mishra A, Singla R, Kaur H, Prabha PK, Patel AP, Medhi B. Prajapat M, et al. Front Aging Neurosci. 2023 Dec 20;15:1296919. doi: 10.3389/fnagi.2023.1296919. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38173557 Free PMC article. - The point mutation UCH-L1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity: implications for post-ischemic neuronal injury.
Liu H, Li W, Rose ME, Hickey RW, Chen J, Uechi GT, Balasubramani M, Day BW, Patel KV, Graham SH. Liu H, et al. Cell Death Dis. 2015 Nov 5;6(11):e1966. doi: 10.1038/cddis.2015.323. Cell Death Dis. 2015. PMID: 26539913 Free PMC article. - Chronic voluntary oral methamphetamine induces deficits in spatial learning and hippocampal protein kinase Mzeta with enhanced astrogliosis and cyclooxygenase-2 levels.
Avila JA, Zanca RM, Shor D, Paleologos N, Alliger AA, Figueiredo-Pereira ME, Serrano PA. Avila JA, et al. Heliyon. 2018 Feb 2;4(2):e00509. doi: 10.1016/j.heliyon.2018.e00509. eCollection 2018 Feb. Heliyon. 2018. PMID: 29560440 Free PMC article. - PACAP27 prevents Parkinson-like neuronal loss and motor deficits but not microglia activation induced by prostaglandin J2.
Shivers KY, Nikolopoulou A, Machlovi SI, Vallabhajosula S, Figueiredo-Pereira ME. Shivers KY, et al. Biochim Biophys Acta. 2014 Sep;1842(9):1707-19. doi: 10.1016/j.bbadis.2014.06.020. Epub 2014 Jun 23. Biochim Biophys Acta. 2014. PMID: 24970746 Free PMC article. - Prostaglandins in the Inflamed Central Nervous System: Potential Therapeutic Targets.
Sheremeta CL, Yarlagadda S, Smythe ML, Noakes PG. Sheremeta CL, et al. Curr Drug Targets. 2024;25(13):885-908. doi: 10.2174/0113894501323980240815113851. Curr Drug Targets. 2024. PMID: 39177131 Free PMC article. Review.
References
- Giles H, Leff P. Prostaglandins. 1988;35:277–300. - PubMed
- Fitzpatrick F A, Wynalda M A. J Biol Chem. 1983;258:11713–11718. - PubMed
- Hirata Y, Hayashi H, Ito S, Kikawa Y, Ishibashi M, Sudo M, Miyazaki H, Fukushima M, Narumiya S, Hayaishi O. J Biol Chem. 1988;263:16619–16625. - PubMed
- Fukushima M. Prostaglandins Leukot Essent Fatty Acids. 1992;47:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous