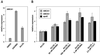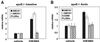Synthetic LXR ligand inhibits the development of atherosclerosis in mice - PubMed (original) (raw)
. 2002 May 28;99(11):7604-9.
doi: 10.1073/pnas.112059299.
Elaine McKilligin, Liming Pei, Michael A Watson, Alan R Collins, Bryan A Laffitte, Mingyi Chen, Grace Noh, Joanne Goodman, Graham N Hagger, Jonathan Tran, Tim K Tippin, Xuping Wang, Aldons J Lusis, Willa A Hsueh, Ronald E Law, Jon L Collins, Timothy M Willson, Peter Tontonoz
Affiliations
- PMID: 12032330
- PMCID: PMC124297
- DOI: 10.1073/pnas.112059299
Synthetic LXR ligand inhibits the development of atherosclerosis in mice
Sean B Joseph et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The nuclear receptors LXRalpha and LXRbeta have been implicated in the control of cholesterol and fatty acid metabolism in multiple cell types. Activation of these receptors stimulates cholesterol efflux in macrophages, promotes bile acid synthesis in liver, and inhibits intestinal cholesterol absorption, actions that would collectively be expected to reduce atherosclerotic risk. However, synthetic LXR ligands have also been shown to induce lipogenesis and hypertriglyceridemia in mice, raising questions as to the net effects of these compounds on the development of cardiovascular disease. We demonstrate here that the nonsteroidal LXR agonist GW3965 has potent antiatherogenic activity in two different murine models. In LDLR(-/-) mice, GW3965 reduced lesion area by 53% in males and 34% in females. A similar reduction of 47% was observed in male apoE(-/-) mice. Long-term (12-week) treatment with LXR agonist had differential effects on plasma lipid profiles in LDLR(-/-) and apoE(-/-) mice. GW3965 induced expression of ATP-binding cassettes A1 and G1 in modified low-density lipoprotein-loaded macrophages in vitro as well as in the aortas of hyperlipidemic mice, suggesting that direct actions of LXR ligands on vascular gene expression are likely to contribute to their antiatherogenic effects. These observations provide direct evidence for an atheroprotective effect of LXR agonists and support their further evaluation as potential modulators of human cardiovascular disease.
Figures
Figure 1
Synthetic LXR ligands are more efficacious than endogenous LXR ligands in lipid-loaded macrophages. (A) Murine thioglycolate-elicited peritoneal macrophages from C57BL/6 mice were treated for 24 h with vehicle (DMSO), 5 μM GW3965, or 50 μg/ml protein AcLDL. (B) Peritoneal macrophages were cultured for 24 h in the presence of 50 μg/ml AcLDL and then treated for an additional 24 h with the indicated concentration of GW3965 or T1317. RNA was isolated and gene expression assayed by real time quantitative PCR. Data are presented as mRNA level relative to vehicle control.
Figure 2
GW3965 inhibits the development of aortic lesions in LDLR−/− mice. Atherosclerotic lesions were quantitated by en face analysis (see Materials and Methods). n = 9 per group.
Figure 3
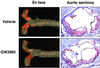
En face and aortic root section analysis of atherosclerosis in LDLR−/− mice.
Figure 4
GW3965 inhibits the development of aortic root lesions in LDLR−/− mice and apoE−/− mice. n = 7 for LDLR−/− and n = 8 for apoE−/−.
Figure 5
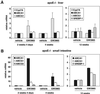
Regulation of LXR target gene expression by GW3965 in liver and intestine in apoE−/− mice. ApoE−/− mice (five animals per group) were treated for the indicated time with either vehicle or 10 mpk GW3965. Gene expression was measured by real-time quantitative PCR (Taqman) assays. Data are presented as mRNA expression relative to vehicle control. SREBP-1, sterol regulatory element binding protein-1.
Figure 6
Expression of ABCA1 protein in macrophages within aortic lesions from LDLR−/− mice. Aortic root sections from LDLR−/− mice after 12 weeks on a high-fat diet were analyzed for ABCA1 and MOMA-2 protein expression by immunostaining.
Figure 7
GW3965 induces expression of ABCA1 and ABCG1 in the atherosclerotic aortas of apoE−/− mice. Aorta and small intestines (n = 3) were isolated from apoE−/− mice of 8 months of age after 4 days of treatment with 10 mpk vehicle or GW3965. Gene expression was measured by real-time quantitative PCR (Taqman) assays. Data are presented as mRNA expression relative to vehicle control.
Similar articles
- Liver X receptor agonist methyl-3β-hydroxy-5α,6α-epoxycholanate attenuates atherosclerosis in apolipoprotein E knockout mice without increasing plasma triglyceride.
Yan W, Zhang T, Cheng J, Zhou X, Qu X, Hu H. Yan W, et al. Pharmacology. 2010;86(5-6):306-12. doi: 10.1159/000321320. Epub 2010 Nov 10. Pharmacology. 2010. PMID: 21071998 - The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions.
Laffitte BA, Joseph SB, Chen M, Castrillo A, Repa J, Wilpitz D, Mangelsdorf D, Tontonoz P. Laffitte BA, et al. Mol Cell Biol. 2003 Mar;23(6):2182-91. doi: 10.1128/MCB.23.6.2182-2191.2003. Mol Cell Biol. 2003. PMID: 12612088 Free PMC article. - The effect of T0901317 on ATP-binding cassette transporter A1 and Niemann-Pick type C1 in apoE-/- mice.
Dai XY, Ou X, Hao XR, Cao DL, Tang YL, Hu YW, Li XX, Tang CK. Dai XY, et al. J Cardiovasc Pharmacol. 2008 May;51(5):467-75. doi: 10.1097/FJC.0b013e31816a5be3. J Cardiovasc Pharmacol. 2008. PMID: 18437096 - Liver X receptors and atherosclerosis.
Lala DS. Lala DS. IDrugs. 2004 Jun;7(6):563-9. IDrugs. 2004. PMID: 15197661 Review. - Liver X receptors (LXR) as therapeutic targets in dyslipidemia.
Bełtowski J. Bełtowski J. Cardiovasc Ther. 2008 Winter;26(4):297-316. doi: 10.1111/j.1755-5922.2008.00062.x. Cardiovasc Ther. 2008. PMID: 19035881 Review.
Cited by
- Drug screen identifies verteporfin as a regulator of lipid metabolism in macrophage foam cells.
Hoeffner N, Paul A, Goo YH. Hoeffner N, et al. Sci Rep. 2023 Nov 9;13(1):19588. doi: 10.1038/s41598-023-46467-4. Sci Rep. 2023. PMID: 37949969 Free PMC article. - Liver X receptor modulates diabetic retinopathy outcome in a mouse model of streptozotocin-induced diabetes.
Hazra S, Rasheed A, Bhatwadekar A, Wang X, Shaw LC, Patel M, Caballero S, Magomedova L, Solis N, Yan Y, Wang W, Thinschmidt JS, Verma A, Li Q, Levi M, Cummins CL, Grant MB. Hazra S, et al. Diabetes. 2012 Dec;61(12):3270-9. doi: 10.2337/db11-1596. Epub 2012 Aug 13. Diabetes. 2012. PMID: 22891211 Free PMC article. - ATF4/TXNIP/REDD1/mTOR signaling mediates the antitumor activities of liver X receptor in pancreatic cancers.
Chen Z, Lai X, Ding H, Zhang A, Sun Y, Ling J, Chiao PJ, Chen Z, Xia X. Chen Z, et al. Cancer Innov. 2022 Jun 30;1(1):55-69. doi: 10.1002/cai2.12. eCollection 2022 Jun. Cancer Innov. 2022. PMID: 38089448 Free PMC article. - Promiscuous activity of the LXR antagonist GSK2033 in a mouse model of fatty liver disease.
Griffett K, Burris TP. Griffett K, et al. Biochem Biophys Res Commun. 2016 Oct 21;479(3):424-428. doi: 10.1016/j.bbrc.2016.09.036. Epub 2016 Sep 25. Biochem Biophys Res Commun. 2016. PMID: 27680310 Free PMC article. - E17110 promotes reverse cholesterol transport with liver X receptor β agonist activity in vitro.
Li N, Wang X, Liu P, Lu D, Jiang W, Xu Y, Si S. Li N, et al. Acta Pharm Sin B. 2016 May;6(3):198-204. doi: 10.1016/j.apsb.2016.03.005. Epub 2016 Apr 5. Acta Pharm Sin B. 2016. PMID: 27175330 Free PMC article.
References
- Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature (London) 1996;383:728–731. - PubMed
- Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. J Biol Chem. 1997;272:3137–3140. - PubMed
- Repa J J, Mangelsdorf D J. Annu Rev Cell Dev Biol. 2000;16:459–481. - PubMed
- Costet P, Luo Y, Wang N, Tall A R. J Biol Chem. 2000;275:28240–28245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL60030/HL/NHLBI NIH HHS/United States
- P01 HL060030/HL/NHLBI NIH HHS/United States
- HL58328/HL/NHLBI NIH HHS/United States
- R01 HL066088/HL/NHLBI NIH HHS/United States
- HL30568/HL/NHLBI NIH HHS/United States
- HL 66088/HL/NHLBI NIH HHS/United States
- P01 HL030568/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous