Cardiomyocyte differentiation of mouse and human embryonic stem cells - PubMed (original) (raw)
Cardiomyocyte differentiation of mouse and human embryonic stem cells
C Mummery et al. J Anat. 2002 Mar.
Abstract
Ischaemic heart disease is the leading cause of morbidity and mortality in the western world. Cardiac ischaemia caused by oxygen deprivation and subsequent oxygen reperfusion initiates irreversible cell damage, eventually leading to widespread cell death and loss of function. Strategies to regenerate damaged cardiac tissue by cardiomyocyte transplantation may prevent or limit post-infarction cardiac failure. We are searching for methods for inducing pluripotent stem cells to differentiate into transplantable cardiomyocytes. We have already shown that an endoderm-like cell line induced the differentiation of embryonal carcinoma cells into immature cardiomyocytes. Preliminary results show that human and mouse embryonic stem cells respond in a similar manner. This study presents initial characterization of these cardiomyocytes and the mouse myocardial infarction model in which we will test their ability to restore cardiac function.
Figures
Fig. 1
Co-cultures of stem cells with the mouse visceral endoderm-like cell line END-2. (a) P19 EC in normal monolayer culture, 3 days after initiation of co-culture with END-2 cells and after 10 days, when beating muscle (B.M.) is evident. (b) mES cell line R1 in monolayer on its normal ‘feeder’ cells (SNL), 3 days after initiation of co-culture and 2 days later, when beating muscle is evident. (c) as (b), with the exception that B.M. is evident on day 7 after aggregation. (d) GCT27X human EC cell line on mouse embryonic fibroblast (MEF) feeder cells, 3 days after initiation of co-culture and after 16 days. No beating muscle is present. (e) hES cells on MEF feeders, 3 days after initiation of END-2 co-culture and beating muscle formed after 11 days.
Fig. 2
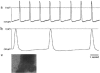
Electrophysiological characteristics of cardiomyocytes from stem cells. Repetitive action potentials recorded from spontaneously beating areas. (a) In mouse P19 EC cell-derived cardiomyocytes. (b) In an aggregate of hES-derived cardiomyocytes. (c) Phase contrast image of the beating area in the hES culture from which the recording showed in (b) was derived. (Note the height of the protruding structure where the beating region is located, 20× objective.)
Fig. 3
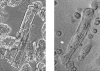
Isolated cardiomyocytes: (a) exhibiting sharp edges and well-defined sarcomeres in contrast with cells cultured for 2 days (b) which had disorganized sarcomeric patterning. (a) is a phase contrast image of multiple cells after isolation and fixation. (b) represents a single cell, digitally magnified 2× compared with (a).
Fig. 4
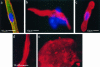
Immunocytochemistry on adult human primary atrial cardiomyocytes and stem cell-derived cardiomyocytes. Primary atrial cardiomyocytes stained positive for sarcomeric proteins including (green) α-actinin, (red) mlc-2a (a) and tropomyosin (b). Cell DNA was stained with (blue) Hoechst to distinguish normal and apoptotic cells. Cells cultured for 2 days had a disorganized tropomyosin sarcomeric patterning and diffuse antibody staining (c). mES-derived cardiomyocytes also show sharp banding when stained with α-actinin (d) but in hES-derived cardiomyocytes α-actinin is diffuse and poorly banded (not shown). (e) shows overall extensive α-actinin staining in hES-derived cardiomyocytes at low magnification.
Fig. 5
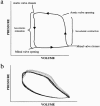
Haemodynamic assessment of left ventricular function in mice. (a) Normal loop representing the relationship between volume and pressure changes in the mouse heart: indicated are the valvular events and stages during one cycle of contraction and relaxation. (b) Pressure volume relationship 4 weeks post-myocardial infarction: note the difference in the shape of the loop and the alterations in both contraction and relaxation.
Similar articles
- Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells.
Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Mummery C, et al. Circulation. 2003 Jun 3;107(21):2733-40. doi: 10.1161/01.CIR.0000068356.38592.68. Epub 2003 May 12. Circulation. 2003. PMID: 12742992 - Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents.
Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Maltsev VA, et al. Circ Res. 1994 Aug;75(2):233-44. doi: 10.1161/01.res.75.2.233. Circ Res. 1994. PMID: 8033337 - Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study.
Ménard C, Hagège AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, Bel A, Messas E, Bissery A, Bruneval P, Desnos M, Pucéat M, Menasché P. Ménard C, et al. Lancet. 2005 Sep 17-23;366(9490):1005-12. doi: 10.1016/S0140-6736(05)67380-1. Lancet. 2005. PMID: 16168783 - Cardiomyocyte differentiation from embryonic and adult stem cells.
Passier R, Mummery C. Passier R, et al. Curr Opin Biotechnol. 2005 Oct;16(5):498-502. doi: 10.1016/j.copbio.2005.08.003. Curr Opin Biotechnol. 2005. PMID: 16099156 Review. - Myocardial regeneration by embryonic stem cell transplantation: present and future trends.
Dai W, Kloner RA. Dai W, et al. Expert Rev Cardiovasc Ther. 2006 May;4(3):375-83. doi: 10.1586/14779072.4.3.375. Expert Rev Cardiovasc Ther. 2006. PMID: 16716098 Review.
Cited by
- Optical mapping of human embryonic stem cell-derived cardiomyocyte graft electrical activity in injured hearts.
Filice D, Dhahri W, Solan JL, Lampe PD, Steele E, Milani N, Van Biber B, Zhu WZ, Valdman TS, Romagnuolo R, Otero-Cruz JD, Hauch KD, Kay MW, Sarvazyan N, Laflamme MA. Filice D, et al. Stem Cell Res Ther. 2020 Sep 25;11(1):417. doi: 10.1186/s13287-020-01919-w. Stem Cell Res Ther. 2020. PMID: 32988411 Free PMC article. - Generation of highly purified human cardiomyocytes from peripheral blood mononuclear cell-derived induced pluripotent stem cells.
Fuerstenau-Sharp M, Zimmermann ME, Stark K, Jentsch N, Klingenstein M, Drzymalski M, Wagner S, Maier LS, Hehr U, Baessler A, Fischer M, Hengstenberg C. Fuerstenau-Sharp M, et al. PLoS One. 2015 May 13;10(5):e0126596. doi: 10.1371/journal.pone.0126596. eCollection 2015. PLoS One. 2015. PMID: 25970162 Free PMC article. - Generating human intestinal tissues from pluripotent stem cells to study development and disease.
Sinagoga KL, Wells JM. Sinagoga KL, et al. EMBO J. 2015 May 5;34(9):1149-63. doi: 10.15252/embj.201490686. Epub 2015 Mar 19. EMBO J. 2015. PMID: 25792515 Free PMC article. Review. - Human embryonic stem cells: an in vitro model to study mechanisms controlling pluripotency in early mammalian development.
Vallier L, Pedersen RA. Vallier L, et al. Stem Cell Rev. 2005;1(2):119-30. doi: 10.1385/SCR:1:2:119. Stem Cell Rev. 2005. PMID: 17142846 Review. - Cardiogenic differentiation and transdifferentiation of progenitor cells.
Reinecke H, Minami E, Zhu WZ, Laflamme MA. Reinecke H, et al. Circ Res. 2008 Nov 7;103(10):1058-71. doi: 10.1161/CIRCRESAHA.108.180588. Circ Res. 2008. PMID: 18988903 Free PMC article. Review.
References
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;200:271–278. - PubMed
- An RH, Davies MP, Doevendans PA, Kubalak SW, Bangalore R, Chien KR, et al. Developmental changes in beta-adrenergic modulation of 1-type Ca2+ channels in embryonic mouse heart. Circ. Res. 1996;78:371–378. - PubMed
- Davies MP, An RH, Doevendans P, Kubalak S, Chien KR, Kass RS. Developmental changes in ionic channel activity in the embryonic murine heart. Circ. Res. 1996;78:15–25. - PubMed
- Doevendans PA, Daemen M, de Muinck E, Smits J. Cardiovascular phenotyping in mice. Cardiovasc. Res. 1998;39:34–49. - PubMed
- Doevendans PA, Kubalak SW, An RH, Becker DK, Chien KR, Kass RS. Differentiation of cardiomyocytes in floating embryoid bodies is comparable to fetal cardiomyocytes. J. Mol. Cell Cardiol. 2000;32:839–851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical