Design and characterization of decoy oligonucleotides containing locked nucleic acids - PubMed (original) (raw)
Design and characterization of decoy oligonucleotides containing locked nucleic acids
Rita Crinelli et al. Nucleic Acids Res. 2002.
Abstract
Transfection of cis-element double-stranded oligonucleotides, referred to as decoy ODNs, has been reported to be a powerful tool that provides a new class of antigene strategies for gene therapy. However, one of the major limitations of the decoy approach is the rapid degradation of phosphodiester oligonucleotides by intracellular nucleases. To date, several DNA analogs have been employed to overcome this issue, but insufficient efficacy and/or specificity have limited their in vivo usefulness. In this paper we have investigated the use of conformationally restricted nucleotides in the design of decoy molecules for nuclear transcription factor kappaB (NF-kappaB). Starting from a synthetic double-stranded oligonucleotide, containing the kappaB consensus binding sequence, we designed a panel of decoy molecules modified to various extents and at various positions with locked nucleic acids (LNAs). Our results indicate that the addition of terminal LNA bases, outside the kappaB sequence, to generate LNA-DNA-LNA co-polymers was sufficient to confer appreciable protection towards nuclease digestion, without interfering with transcription factor binding. Conversely, insertion of LNA substitutions in the context of the kappaB-binding site resulted in further increased stability, but caused a loss of affinity of NF-kappaB for the target sequence. However, our results also indicate that this latter effect was apparently dependent not only on the extent but also on strand positioning of the internal LNA substitutions. This observation is of great importance since it provides evidence for the possibility of tuning DNA-LNA duplexes with internal LNAs into decoy agents with improved features in terms of biological stability and inhibitory effect.
Figures
Figure 1
Schematic representation of the PRDII κB-binding consensus sequence and of the derived LNA ODNs. Schematic representation of the PRDII κB element showing binding of the NF-κB p50/p65 heterodimer in the outer GC-rich sequence and of HMG I proteins in the core AT-rich domain. Both NF-κB and HMG I can separately bind to the PRDII sequence. In the context of the IFN-β promoter, HMG I functions as an architectural factor facilitating the assembly of transcriptionally active nucleoprotein complexes (40,41). Dots represent hydrogen bonds mediating base-specific contacts between p50 and p65 subunits and the κB site, according to evidence obtained in crystallographic studies (39). LNA ODNs were synthesized on the basis of the κB sequence contained in the PRDII domain of the IFN-β promoter which has been extended at both terminal ends with unrelated extra sequences of 5 nt (in gray). LNA substitutions are indicated in bold upper case letters.
Figure 2
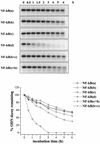
Susceptibility to DNase I degradation of LNA-modified ODNs. LNA-modified [NF-κB(a), (b), (c), (c+b) and (b+c)] and control phosphodiester [NF-κB(d)] decoy molecules were incubated for different lengths of time, as indicated, with 0.5 U/ml DNase I and then submitted to electrophoretic separation on 2.5% (w/v) agarose gels. Detection and quantitation of the ethidium bromide stained bands were performed in a Molecular Analyst. Volume densities of the bands are expressed as percent ODN decoy remaining with respect to the relative time zero value and are shown as line graphs.
Figure 3
Susceptibility to BAL-31 nuclease degradation of LNA-modified ODNs. LNA-modified [NF-κB(a), (b), (c), (c+b) and (b+c)] and control phosphodiester [NF-κB(d)] decoy molecules were incubated for different lengths of time, as indicated, with 0.5 U/ml BAL-31 and then submitted to electrophoretic separation on 2.5% (w/v) agarose gels. Detection and quantitation of the ethidium bromide stained bands were performed in a Molecular Analyst. Volume densities of the bands are expressed as percent ODN decoy remaining with respect to the relative time zero value and are shown as line graphs.
Figure 4
Direct binding of NF-κB to radiolabeled LNA-modified and phosphodiester probes and supershift analysis of the TNF-induced protein–PRDII complex. (A) Nuclear extracts obtained from TNF-stimulated NIH 3T3 cells were incubated with radiolabeled NF-κB(a) (lanes 1–3), NF-κB(b) (lanes 4–6), NF-κB(c) (lanes 7–9), NF-κB(d) (lanes 10–12), NF-κB(c+b) (lanes 13–15), NF-κB (b+c) (lanes 16–18) and PRDII (lanes 19–21) probes and analyzed by EMSA. Specificity of binding was assessed by competition with a cold wild-type Ig-κB probe (Ig-κB wt) (lanes 2, 5, 8, 11, 14, 17 and 20) or a mutated form of Ig-κB (Ig-κB mut) which ablates NF-κB binding (lanes 3, 6, 9, 12, 15, 18 and 21). Due to the different specific activities of the probes (see Materials and Methods) the data are only qualitative and not quantitative. (B) Nuclear extracts prepared from TNF-stimulated NIH 3T3 cells were incubated with the PRDII probe in the absence (lane 1) or presence of the indicated NF-κB subunit-specific antisera (lanes 2 and 3). The resulting complexes were resolved on a 5% non-denaturing gel and detected in a Molecular Imager.
Figure 5
Competition of LNA and phosphodiester ODNs for binding of NF-κB to radiolabeled PRDII probe. Competition experiments were performed by incubating crude nuclear extracts together with the [32P]PRDII probe (1.2 nM) and the indicated unlabeled competitor. NF-κB–PRDII complex formation in the presence of increasing competitor concentrations was analyzed by EMSA and quantitated in a Molecular Imager. Data were expressed as percent binding relative to the level of NF-κB–radioligand complex formation in the absence of the competitor. IC50 values for each type of competitor were estimated by plotting these data as a function of log10 of the competitor concentration (nM) and fitting to a dose-response curve. Curve fitting was performed with Origin 4.1 software. Fifty percent inhibitory concentrations (nM) ± SE are indicated.
Similar articles
- Transcription factor decoy oligonucleotides modified with locked nucleic acids: an in vitro study to reconcile biostability with binding affinity.
Crinelli R, Bianchi M, Gentilini L, Palma L, Sørensen MD, Bryld T, Babu RB, Arar K, Wengel J, Magnani M. Crinelli R, et al. Nucleic Acids Res. 2004 Mar 29;32(6):1874-85. doi: 10.1093/nar/gkh503. Print 2004. Nucleic Acids Res. 2004. PMID: 15051810 Free PMC article. - Locked nucleic acids (LNA): versatile tools for designing oligonucleotide decoys with high stability and affinity.
Crinelli R, Bianchi M, Gentilini L, Palma L, Magnani M. Crinelli R, et al. Curr Drug Targets. 2004 Nov;5(8):745-52. doi: 10.2174/1389450043345083. Curr Drug Targets. 2004. PMID: 15578954 Review. - Binding force measurement of NF-κB-ODNs interaction: an AFM based decoy and drug testing tool.
Menotta M, Crinelli R, Carloni E, Mussi V, Valbusa U, Magnani M. Menotta M, et al. Biosens Bioelectron. 2011 Oct 15;28(1):158-65. doi: 10.1016/j.bios.2011.07.013. Epub 2011 Jul 18. Biosens Bioelectron. 2011. PMID: 21802937 - LNA/DNA chimeric oligomers mimic RNA aptamers targeted to the TAR RNA element of HIV-1.
Darfeuille F, Hansen JB, Orum H, Di Primo C, Toulmé JJ. Darfeuille F, et al. Nucleic Acids Res. 2004 Jun 4;32(10):3101-7. doi: 10.1093/nar/gkh636. Print 2004. Nucleic Acids Res. 2004. PMID: 15181175 Free PMC article. - Promising nucleic acid analogs and mimics: characteristic features and applications of PNA, LNA, and morpholino.
Karkare S, Bhatnagar D. Karkare S, et al. Appl Microbiol Biotechnol. 2006 Aug;71(5):575-86. doi: 10.1007/s00253-006-0434-2. Epub 2006 May 9. Appl Microbiol Biotechnol. 2006. PMID: 16683135 Review.
Cited by
- Transcription factor decoy: a pre-transcriptional approach for gene downregulation purpose in cancer.
Rad SM, Langroudi L, Kouhkan F, Yazdani L, Koupaee AN, Asgharpour S, Shojaei Z, Bamdad T, Arefian E. Rad SM, et al. Tumour Biol. 2015 Jul;36(7):4871-81. doi: 10.1007/s13277-015-3344-z. Epub 2015 Apr 4. Tumour Biol. 2015. PMID: 25835969 Review. - Allele-specific KRT1 expression is a complex trait.
Tao H, Cox DR, Frazer KA. Tao H, et al. PLoS Genet. 2006 Jun;2(6):e93. doi: 10.1371/journal.pgen.0020093. Epub 2006 Jun 9. PLoS Genet. 2006. PMID: 16789827 Free PMC article. - Coarse-Grained Brownian Dynamics Simulations of the 10-23 DNAzyme.
Kenward M, Dorfman KD. Kenward M, et al. Biophys J. 2009 Nov 18;97(10):2785-93. doi: 10.1016/j.bpj.2009.09.003. Biophys J. 2009. PMID: 19917233 Free PMC article. - Promising strategies employing nucleic acids as antimicrobial drugs.
Moreira L, Guimarães NM, Santos RS, Loureiro JA, Pereira MC, Azevedo NF. Moreira L, et al. Mol Ther Nucleic Acids. 2024 Jan 18;35(1):102122. doi: 10.1016/j.omtn.2024.102122. eCollection 2024 Mar 12. Mol Ther Nucleic Acids. 2024. PMID: 38333674 Free PMC article. Review. - Nucleic acid-based approaches to STAT inhibition.
Sen M, Grandis JR. Sen M, et al. JAKSTAT. 2012 Oct 1;1(4):285-91. doi: 10.4161/jkst.22312. JAKSTAT. 2012. PMID: 24058785 Free PMC article. Review.
References
- Papavassiliou A.G. (1998) Transcription-factor-modulating agents: precision and selectivity in drug design. Mol. Med. Today, 4, 358–366. - PubMed
- Morishita R., Higaki,J., Tomita,N. and Ogihara,T. (1998) Application of transcription factor “decoy” strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ. Res., 82, 1023–1028. - PubMed
- Cho-Chung Y.S., Park,Y.G. and Lee,Y.N. (1999) Oligonucleotides as transcription factor decoys. Curr. Opin. Mol. Ther., 1, 386–392. - PubMed
- Bielinska A., Shivdasani,R.A., Zhang,L. and Nabel,G.J. (1990) Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science, 250, 997–1000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources