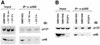Sequential binding of UV DNA damage binding factor and degradation of the p48 subunit as early events after UV irradiation - PubMed (original) (raw)
Sequential binding of UV DNA damage binding factor and degradation of the p48 subunit as early events after UV irradiation
Vesna Rapić-Otrin et al. Nucleic Acids Res. 2002.
Abstract
The UV-damaged DNA binding protein complex (UV-DDB) is implicated in global genomic nucleotide excision repair (NER) in mammalian cells. The complex consists of a heterodimer of p127 and p48. UV-DDB is defective in one complementation group (XP-E) of the heritable, skin cancer-prone disorder xeroderma pigmentosum. Upon UV irradiation of primate cells, UV-DDB associates tightly with chromatin, concomitant with the loss of extractable binding activity. We report here that an early event after UV, but not ionizing, radiation is the transient dose-dependent degradation of the small subunit, p48. Treatment of human cells with the proteasomal inhibitor NIP-L3VS blocks this UV-induced degradation of p48. In XP-E cell lines with impaired UV-DDB binding, p48 is resistant to degradation. UV-mediated degradation of p48 occurs independently of the expression of p53 and the cell's proficiency for NER, but recovery of p48 levels at later times (12 h and thereafter) is dependent upon the capacity of the cell to repair non-transcribed DNA. In addition, we find that the p127 subunit of UV-DDB binds in vivo to p300, a histone acetyltransferase. The data support a functional connection between UV-DDB binding activity, proteasomal degradation of p48 and chromatin remodeling during early steps of NER.
Figures
Figure 1
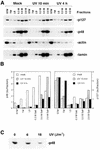
A tight association with chromatin, followed by a dose-dependent degradation of p48, is an early response to UV irradiation. (A) Immunodetection of p48, p127, actin and lamin B in nuclear fractions prepared from unirradiated and irradiated (12 J/m2) GM01953 cells and harvested 10 min and 4 h after treatment. An aliquot of 30 µg protein was loaded for each fraction: STM wash, Triton wash (TW), low Mg2+ wash (LM), 0.3 M salt extract (0.3 M), 0.5 M salt extract (0.5 M) and 2.0 M salt extract (2.0 M), prepared as described in Materials and Methods. (B) Plots of data derived from (A), expressed as total amounts of p48 and p127 in each fraction in arbitrary units (au). (C) TC-7 cells were irradiated with 6 or 18 J/m2 and cell lysates were prepared immediately after UV treatment and immunoprecipitated with guinea pig anti-p48 antibody. The immunoprecipitates were analyzed by immunoblotting using rabbit anti-p48 antibody.
Figure 2
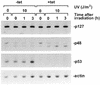
UV but not IR irradiation transiently decreases the level of p48. TC-7 (A–C) and GM01953 (D) cells were exposed to UV (10 J/m2) and IR (15 Gy) irradiation, respectively, and harvested at the indicated time points. The levels of p48, p127, p53 and actin were measured by immunoblotting of whole cell extracts (50 µg) (A and D) or cytosol and nuclear extracts (75 µg) (p127 and p48 only) (B). The same membrane was cut into two or three strips and each strip was probed with specific antibody. The strip probed for p48 was stripped and incubated with anti-actin antibodies, as the internal control. (C) To correlate the amount of p48 protein with UV-DDB binding activity, the nuclear and cytosol fractions (5 µg) were incubated under standard conditions (39) with labeled UV-damaged, double-stranded 60mer DNA oligonucleotides. Free and bound complexes were separated on a non-denaturing 6% polyacrylamide gel.
Figure 2
UV but not IR irradiation transiently decreases the level of p48. TC-7 (A–C) and GM01953 (D) cells were exposed to UV (10 J/m2) and IR (15 Gy) irradiation, respectively, and harvested at the indicated time points. The levels of p48, p127, p53 and actin were measured by immunoblotting of whole cell extracts (50 µg) (A and D) or cytosol and nuclear extracts (75 µg) (p127 and p48 only) (B). The same membrane was cut into two or three strips and each strip was probed with specific antibody. The strip probed for p48 was stripped and incubated with anti-actin antibodies, as the internal control. (C) To correlate the amount of p48 protein with UV-DDB binding activity, the nuclear and cytosol fractions (5 µg) were incubated under standard conditions (39) with labeled UV-damaged, double-stranded 60mer DNA oligonucleotides. Free and bound complexes were separated on a non-denaturing 6% polyacrylamide gel.
Figure 2
UV but not IR irradiation transiently decreases the level of p48. TC-7 (A–C) and GM01953 (D) cells were exposed to UV (10 J/m2) and IR (15 Gy) irradiation, respectively, and harvested at the indicated time points. The levels of p48, p127, p53 and actin were measured by immunoblotting of whole cell extracts (50 µg) (A and D) or cytosol and nuclear extracts (75 µg) (p127 and p48 only) (B). The same membrane was cut into two or three strips and each strip was probed with specific antibody. The strip probed for p48 was stripped and incubated with anti-actin antibodies, as the internal control. (C) To correlate the amount of p48 protein with UV-DDB binding activity, the nuclear and cytosol fractions (5 µg) were incubated under standard conditions (39) with labeled UV-damaged, double-stranded 60mer DNA oligonucleotides. Free and bound complexes were separated on a non-denaturing 6% polyacrylamide gel.
Figure 3
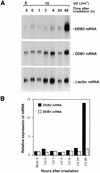
Early suppression of p48 protein is not transcriptionally controlled. (A) Expression of p48 and p127 transcripts at various times after irradiation was examined by northern blot analysis. The experimental protocol was as in Figure 2A. The blot was first hybridized with random primed probes containing an isolated coding region of p48 cDNA and, after stripping, the blot was hybridized sequentially with probes for p127 and β-actin. (B) Autoradiograms were quantitated (NIH Image 1.62) and relative expression of p127 mRNA or p48 mRNA was normalized to the level of β-actin in each sample. Expression level of the mRNAs in unirradiated samples (mock 0) was designated 1.
Figure 4
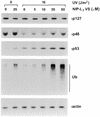
A proteasomal inhibitor blocks UV-induced degradation of p48. Normal human lymphoblastoid cells (GM01953) were pretreated with the specific proteasomal inhibitor NIP-L3VS at the indicated concentrations for 1 h and then subjected to UV irradiation. Fresh medium with NIP-L3VS was used for further post-UV incubation for 3 h. The levels of p127, p48, p53 and total ubiquitinated proteins were detected in whole cell extracts (75 µg) using the specific antibodies. The membrane was probed with anti-actin antibodies, as the internal control.
Figure 5
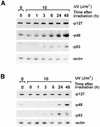
Mutated p48 is resistant to UV-induced degradation. (A) Fibroblasts from an XP-E patient (XP82TO) harboring a K244E substitution in p48, which impaired UV-DDB binding activity, and (B) normal fibroblasts (GM05757) were subjected to the same experimental protocol as in Figure 2. The whole cell extracts (70 µg) were used for western blot analysis.
Figure 6
Defective global genome repair affects the post-UV recovery of p48. (A and B) Normal human primary fibroblasts and various XP and CS repair-deficient primary fibroblasts were irradiated with the following UV doses: 10 J/m2 (normal fibroblasts, GM05757; CS-A, GM01865; XP-E, GM02415; XP-V, XP7Br) or 6 J/m2 (XP-A, GM05509; XP-B, GM13025; XP-C, MG00676; XP-D, GM03248; XP-F, GM04313). (C) A normal SV40 transformed cell line (GM00637), XP-A revertant (XP129) and its parental cell (XP12RO) were irradiated at 6, 3 and 3 J/m2, respectively. The wce from unirradiated and irradiated cells were prepared at the indicated time points after irradiation and 60 µg were used for immunoblot analysis.
Figure 7
UV-induced degradation of p48 is not mediated through p53. TR9-7 cells, with a stably integrated Tet-regulated wild-type p53 gene, were grown without (–Tet) or with 2 µg/ml tetracycline (+Tet) for 3 days and then subjected to UV irradiation. The cells were treated with or without tetracycline for the indicated times after irradiation, after which wce were prepared and 75 µg used for western blot analysis.
Figure 8
. Interaction of UV-DDB with p300 in vivo occurs through the p127 subunit. (A) GM01953 cells were irradiated with 15 J/m2 and cell lysates prepared 10 min and 2 h after treatment were immunoprecipitated with anti-p300 antibody. The co-immunoprecipitates were analyzed by immunoblotting using anti-p127 and anti-p48 antibodies. (B) The lysates from normal and two UV-DDB binding-deficient XP-E cell lines (GM01646 and XP23PV) were immunoprecipitated in the same way as in (A).
Similar articles
- True XP group E patients have a defective UV-damaged DNA binding protein complex and mutations in DDB2 which reveal the functional domains of its p48 product.
Rapić-Otrin V, Navazza V, Nardo T, Botta E, McLenigan M, Bisi DC, Levine AS, Stefanini M. Rapić-Otrin V, et al. Hum Mol Genet. 2003 Jul 1;12(13):1507-22. doi: 10.1093/hmg/ddg174. Hum Mol Genet. 2003. PMID: 12812979 - The p48 subunit of the damaged-DNA binding protein DDB associates with the CBP/p300 family of histone acetyltransferase.
Datta A, Bagchi S, Nag A, Shiyanov P, Adami GR, Yoon T, Raychaudhuri P. Datta A, et al. Mutat Res. 2001 Jul 12;486(2):89-97. doi: 10.1016/s0921-8777(01)00082-9. Mutat Res. 2001. PMID: 11425514 - Translocation of a UV-damaged DNA binding protein into a tight association with chromatin after treatment of mammalian cells with UV light.
Otrin VR, McLenigan M, Takao M, Levine AS, Protić M. Otrin VR, et al. J Cell Sci. 1997 May;110 ( Pt 10):1159-68. doi: 10.1242/jcs.110.10.1159. J Cell Sci. 1997. PMID: 9191040 - Xeroderma pigmentosum complementation group E and UV-damaged DNA-binding protein.
Tang J, Chu G. Tang J, et al. DNA Repair (Amst). 2002 Aug 6;1(8):601-16. doi: 10.1016/s1568-7864(02)00052-6. DNA Repair (Amst). 2002. PMID: 12509284 Free PMC article. Review. - Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair.
Sugasawa K. Sugasawa K. DNA Repair (Amst). 2016 Aug;44:110-117. doi: 10.1016/j.dnarep.2016.05.015. Epub 2016 May 20. DNA Repair (Amst). 2016. PMID: 27264556 Review.
Cited by
- Orchestral maneuvers at the damaged sites in nucleotide excision repair.
Alekseev S, Coin F. Alekseev S, et al. Cell Mol Life Sci. 2015 Jun;72(11):2177-86. doi: 10.1007/s00018-015-1859-5. Epub 2015 Feb 15. Cell Mol Life Sci. 2015. PMID: 25681868 Free PMC article. Review. - A novel cis-acting element from the 3'UTR of DNA damage-binding protein 2 mRNA links transcriptional and post-transcriptional regulation of gene expression.
Melanson BD, Cabrita MA, Bose R, Hamill JD, Pan E, Brochu C, Marcellus KA, Zhao TT, Holcik M, McKay BC. Melanson BD, et al. Nucleic Acids Res. 2013 Jun;41(11):5692-703. doi: 10.1093/nar/gkt279. Epub 2013 Apr 19. Nucleic Acids Res. 2013. PMID: 23605047 Free PMC article. - Imaging the response to DNA damage in heterochromatin domains reveals core principles of heterochromatin maintenance.
Fortuny A, Chansard A, Caron P, Chevallier O, Leroy O, Renaud O, Polo SE. Fortuny A, et al. Nat Commun. 2021 Apr 23;12(1):2428. doi: 10.1038/s41467-021-22575-5. Nat Commun. 2021. PMID: 33893291 Free PMC article. - The emerging role of deubiquitination in nucleotide excision repair.
Zhang L, Gong F. Zhang L, et al. DNA Repair (Amst). 2016 Aug;44:118-122. doi: 10.1016/j.dnarep.2016.05.035. Epub 2016 Jun 2. DNA Repair (Amst). 2016. PMID: 27316462 Free PMC article. Review. - Overexpression of DDB2 enhances the sensitivity of human ovarian cancer cells to cisplatin by augmenting cellular apoptosis.
Barakat BM, Wang QE, Han C, Milum K, Yin DT, Zhao Q, Wani G, Arafa el-SA, El-Mahdy MA, Wani AA. Barakat BM, et al. Int J Cancer. 2010 Aug 15;127(4):977-88. doi: 10.1002/ijc.25112. Int J Cancer. 2010. PMID: 20013802 Free PMC article.
References
- Bootsma D., Kraemer,K.H., Cleaver,J.E. and Hoeijmakers,J.H.J. (1998) Nucleotide excision repair syndromes: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. In Vogelstein,B. and Kinzler,K.W. (eds), The Genetic Basis of Human Cancer. McGraw-Hill, New York, NY, pp. 245–274.
- de Boer J. and Hoeijmakers,J.H. (2000) Nucleotide excision repair and human syndromes. Carcinogenesis, 21, 453–460. - PubMed
- Wood R.D., Mitchell,M., Sgouros,J. and Lindahl,T. (2001) Human DNA repair genes. Science, 291, 1284–1289. - PubMed
- de Laat W.L., Jaspers,N.G. and Hoeijmakers,J.H. (1999) Molecular mechanism of nucleotide excision repair. Genes Dev., 13, 768–785. - PubMed
- Batty D. and Wood,R.D. (2000) Damage recognition in nucleotide excision repair of DNA. Gene, 241, 193–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous