Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development - PubMed (original) (raw)
Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development
Geraldine Dawson et al. Child Dev. 2002 May-Jun.
Abstract
This study utilized electroencephalographic recordings to examine whether young children with autism spectrum disorder (ASD) have impaired face recognition ability. High-density brain event-related potentials (ERPs) were recorded to photos of the child's mother's face versus an unfamiliar female face and photos of a favorite versus an unfamiliar toy from children with ASD, children with typical development, and children with developmental delay, all 3 to 4 years of age (N = 118). Typically developing children showed ERP amplitude differences in two components, P400 and Nc, to a familiar versus an unfamiliar face, and to a familiar versus an unfamiliar object. In contrast, children with ASD failed to show differences in ERPs to a familiar versus an unfamiliar face, but they did show P400 and Nc amplitude differences to a familiar versus an unfamiliar object. Developmentally delayed children showed significant ERP amplitude differences for the positive slow wave for both faces and objects. These data suggest that autism is associated with face recognition impairment that is manifest early in life.
Figures
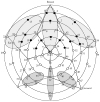
Figure 1
Electrode groups over which data were averaged for each component (reference electrode during recording at location Cz). The Nc component is shown in the light-shaded areas (top). The P400 component is shown in the dark-shaded areas (bottom). Electrodes that are shaded black indicate the slow-wave component.
Figure 2
Voltage maps of event-related potentials to unfamiliar faces at 450 ms for children with (A) autism spectrum disorder, (B) typical development, and (C) developmental delay.
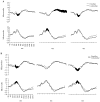
Figure 3
Averaged event-related potential waveforms at the anterior (top) and posterior (bottom), right hemisphere, midline, and left hemisphere scalp locations for familiar and unfamiliar (A) faces and (B) objects for children with typical development. Areas in which significant differences were found for familiar versus unfamiliar stimuli are shaded in black.
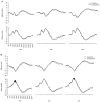
Figure 4
Averaged event-related potential waveforms at the anterior (top) and posterior (bottom), right hemisphere, midline, and left hemisphere scalp locations for familiar and unfamiliar (A) faces and (B) objects for children with autism spectrum disorder. Areas in which significant differences were found for familiar versus unfamiliar stimuli are shaded in black.
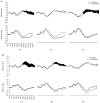
Figure 5
Averaged event-related potential waveforms at the anterior (top) and posterior (bottom), right hemisphere, midline, and left hemisphere scalp locations for familiar and unfamiliar (A) faces and (B) objects for children with developmental delay. Areas in which significant differences were found for familiar versus unfamiliar stimuli are shaded in black.
Figure 6
Voltage maps of event-related potentials to unfamiliar objects at 490 ms for children with (A) autism spectrum disorder, (B) typical development, and (C) developmental delay.
Similar articles
- Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism.
McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. McCleery JP, et al. Biol Psychiatry. 2009 Nov 15;66(10):950-7. doi: 10.1016/j.biopsych.2009.07.031. Epub 2009 Sep 18. Biol Psychiatry. 2009. PMID: 19765688 Free PMC article. - Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion.
Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Dawson G, et al. Dev Sci. 2004 Jun;7(3):340-59. doi: 10.1111/j.1467-7687.2004.00352.x. Dev Sci. 2004. PMID: 15595374 - Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development.
Webb SJ, Jones EJ, Merkle K, Venema K, Greenson J, Murias M, Dawson G. Webb SJ, et al. Child Dev. 2011 Nov-Dec;82(6):1868-86. doi: 10.1111/j.1467-8624.2011.01656.x. Epub 2011 Oct 17. Child Dev. 2011. PMID: 22004249 Free PMC article. - Processing of novel and familiar faces in infants at average and high risk for autism.
Key AP, Stone WL. Key AP, et al. Dev Cogn Neurosci. 2012 Apr;2(2):244-55. doi: 10.1016/j.dcn.2011.12.003. Epub 2011 Dec 21. Dev Cogn Neurosci. 2012. PMID: 22483074 Free PMC article. - Development of face-sensitive event-related potentials during infancy: a review.
de Haan M, Johnson MH, Halit H. de Haan M, et al. Int J Psychophysiol. 2003 Dec;51(1):45-58. doi: 10.1016/s0167-8760(03)00152-1. Int J Psychophysiol. 2003. PMID: 14629922 Review.
Cited by
- Effects of eye gaze cues provided by the caregiver compared to a stranger on infants' object processing.
Hoehl S, Wahl S, Michel C, Striano T. Hoehl S, et al. Dev Cogn Neurosci. 2012 Jan;2(1):81-9. doi: 10.1016/j.dcn.2011.07.015. Epub 2011 Jul 30. Dev Cogn Neurosci. 2012. PMID: 22682729 Free PMC article. - Neonatal serotonin depletion alters behavioral responses to spatial change and novelty.
Hohmann CF, Walker EM, Boylan CB, Blue ME. Hohmann CF, et al. Brain Res. 2007 Mar 30;1139:163-77. doi: 10.1016/j.brainres.2006.12.095. Epub 2007 Jan 17. Brain Res. 2007. PMID: 17296168 Free PMC article. - Impaired face processing in autism: fact or artifact?
Jemel B, Mottron L, Dawson M. Jemel B, et al. J Autism Dev Disord. 2006 Jan;36(1):91-106. doi: 10.1007/s10803-005-0050-5. J Autism Dev Disord. 2006. PMID: 16477517 Review. - Face processing in Pervasive Developmental Disorder (PDD): the roles of expertise and spatial frequency.
Boeschoten MA, Kenemans JL, van Engeland H, Kemner C. Boeschoten MA, et al. J Neural Transm (Vienna). 2007;114(12):1619-29. doi: 10.1007/s00702-007-0780-y. Epub 2007 Jul 18. J Neural Transm (Vienna). 2007. PMID: 17636350 - Atypical neural modulation in the right prefrontal cortex during an inhibitory task with eye gaze in autism spectrum disorder as revealed by functional near-infrared spectroscopy.
Ikeda T, Hirai M, Sakurada T, Monden Y, Tokuda T, Nagashima M, Shimoizumi H, Dan I, Yamagata T. Ikeda T, et al. Neurophotonics. 2018 Jul;5(3):035008. doi: 10.1117/1.NPh.5.3.035008. Epub 2018 Sep 5. Neurophotonics. 2018. PMID: 30211250 Free PMC article.
References
- Aggleton JP. The functional effects of amygdala lesions in humans: A comparison with findings from monkeys. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 485–503.
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Research. 1980;190:347–368. - PubMed
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in the occipitotemporal cortex by face and non-face stimuli. Cerebral Cortex. 1999;9:415–430. - PubMed
- Amaral DG, Price JL, Pitkanen A, Carmichael T. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 1–66.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources