Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep - PubMed (original) (raw)
Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep
Jun Lu et al. J Neurosci. 2002.
Abstract
We found previously that damage to a cluster of sleep-active neurons (Fos-positive during sleep) in the ventrolateral preoptic nucleus (VLPO) decreases non-rapid eye movement (NREM) sleep in rats, whereas injury to the sleep-active cells extending dorsally and medially from the VLPO cluster (the extended VLPO) diminishes REM sleep. These results led us to examine whether neurons in the extended VLPO are activated during REM sleep and the connectivity of these neurons with pontine sites implicated in producing REM sleep: the laterodorsal tegmental nucleus (LDT), dorsal raphe nucleus (DRN), and locus ceruleus (LC). After periods of dark exposure that triggered enrichment of REM sleep, the number of Fos-positive cells in the extended VLPO was highly correlated with REM but not NREM sleep. In contrast, the number of Fos-positive cells in the VLPO cluster was correlated with NREM but not REM sleep. Sixty percent of sleep-active cells in the extended VLPO and 90% of sleep-active cells in the VLPO cluster in dark-treated animals contained galanin mRNA. Retrograde tracing from the LDT, DRN, and LC demonstrated more labeled cells in the extended VLPO than the VLPO cluster, and 50% of these in the extended VLPO were sleep-active. Anterograde tracing showed that projections from the extended VLPO and VLPO cluster targeted the cell bodies and dendrites of DRN serotoninergic neurons and LC noradrenergic neurons but were not apposed to cholinergic neurons in the LDT. The connections and physiological activity of the extended VLPO suggest a specialized role in the regulation of REM sleep.
Figures
Fig. 1.
A pair of photomicrographs showing the distributions of Fos-ir cells in the extended VLPO and VLPO cluster in an animal exposed to light (12% REM sleep, A) and in an animal exposed to dark treatment (30% REM sleep, B) during the early part of the sleep cycle. The counting boxes used for the VLPO cluster and the dorsal and medial extended VLPO are shown in B. These sections are approximately at the level of AP −0.5 in Paxinos and Watson (1986).OC, Optic chiasm.
Fig. 2.
Correlation (illustrated by solid line) of the number of Fos-ir cells in the extended VLPO and VLPO cluster in light- and dark-treated animals with the amounts of REM sleep or NREM sleep that the animals experienced during the hour before perfusion.
Fig. 3.
Photomicrographs showing dual labeling of Fos (brown immunostaining) and galanin mRNA (black silver grains representing in situ hybridization) in the extended VLPO and VLPO cluster. Arrowheads indicate double-labeled cells. Many Fos-ir cells in the extended VLPO and particularly in the VLPO cluster contain galanin mRNA. _A_shows the VLPO complex with boxes that are shown at higher magnification in B–D. Note that these fields are not equivalent to the counting boxes shown in Figure 1.OC, Optic chiasm.
Fig. 4.
Camera lucida drawings showing selected injection sites (black solid and dashed lines) of CTB in the region of the DRN (A), LDT (B), and LC (C) as well as four injection sites of PHA-L in the VLPO region (D). Gray shading shows borders of key nuclei;gray lines show other brain structures. Br, Barrington's nucleus; HDB, horizontal limb of the nucleus of the diagonal band of Broca; mlf, medial longitudinal fasciculus; MnRn, median raphe nucleus;OC, optic chiasm; scp, superior cerebellar peduncle; VTg, ventral tegmental nucleus.
Fig. 5.
Dual labeling of CTB retrograde transport (brown) and Fos expression (black) in cells within the extended VLPO and VLPO cluster (B,E, H) after the injections of CTB into the central DRN (A, experiment R2049), LDT (D, experiment R1969), and LC (G, experiment R2219), respectively, in the rats exposed to 3 hr of darkness. In B, E, and H, the VLPO cluster is identified by a large arrow and the extended VLPO by a large double-headed arrow. The_rectangular boxes_ identify the fields that are magnified in C, F, and I, respectively. Double-labeled cells in the extended VLPO and VLPO cluster are indicated by small arrows and single CTB labeled cells are indicated by small arrowheads.
Fig. 6.
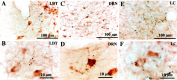
Photomicrographs showing the relationships of anterogradely labeled efferent axons (black) from the extended VLPO and VLPO cluster with neurons in the DRN, LDT, and LC that are stained (brown) for serotonin, choline acetyltransferase, or tyrosine hydroxylase, respectively. In_A_, the efferent terminals concentrate dorsal to the cluster of the cholinergic cells in the LDT. Occasionally we found that terminal boutons were near choline acetyltransferase-immunoreactive neurons such as in B; however careful observation indicated that such boutons were not on the same plane as the cholinergic cell body. In C and D, efferent terminal boutons clearly appose serotonin-immunoreactive neurons in the DRN. E and F show labeled efferent axons apposing tyrosine hydroxylase-immunoreactive neurons in the LC.
Similar articles
- Distribution of hypocretin-containing neurons in the lateral hypothalamus and C-fos-immunoreactive neurons in the VLPO.
Wagner D, Salin-Pascual R, Greco MA, Shiromani PJ. Wagner D, et al. Sleep Res Online. 2000;3(1):35-42. Sleep Res Online. 2000. PMID: 11382898 - Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep.
Lu J, Greco MA, Shiromani P, Saper CB. Lu J, et al. J Neurosci. 2000 May 15;20(10):3830-42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. J Neurosci. 2000. PMID: 10804223 Free PMC article. - [Neurochemical mechanisms of sleep regulation].
[No authors listed] [No authors listed] Glas Srp Akad Nauka Med. 2009;(50):97-109. Glas Srp Akad Nauka Med. 2009. PMID: 20666118 Review. Serbian. - Hypothalamic control of sleep.
Szymusiak R, Gvilia I, McGinty D. Szymusiak R, et al. Sleep Med. 2007 Jun;8(4):291-301. doi: 10.1016/j.sleep.2007.03.013. Epub 2007 Apr 30. Sleep Med. 2007. PMID: 17468047 Review.
Cited by
- The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions.
Inutsuka A, Yamanaka A. Inutsuka A, et al. Front Endocrinol (Lausanne). 2013 Mar 6;4:18. doi: 10.3389/fendo.2013.00018. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23508038 Free PMC article. - Sleep disturbance induces neuroinflammation and impairment of learning and memory.
Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z. Zhu B, et al. Neurobiol Dis. 2012 Dec;48(3):348-55. doi: 10.1016/j.nbd.2012.06.022. Epub 2012 Jul 7. Neurobiol Dis. 2012. PMID: 22776332 Free PMC article. - Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action.
Laque A, Zhang Y, Gettys S, Nguyen TA, Bui K, Morrison CD, Münzberg H. Laque A, et al. Am J Physiol Endocrinol Metab. 2013 May 1;304(9):E999-1011. doi: 10.1152/ajpendo.00643.2012. Epub 2013 Mar 12. Am J Physiol Endocrinol Metab. 2013. PMID: 23482448 Free PMC article. - Glucocorticoid receptors in the locus coeruleus mediate sleep disorders caused by repeated corticosterone treatment.
Wang ZJ, Zhang XQ, Cui XY, Cui SY, Yu B, Sheng ZF, Li SJ, Cao Q, Huang YL, Xu YP, Zhang YH. Wang ZJ, et al. Sci Rep. 2015 Mar 24;5:9442. doi: 10.1038/srep09442. Sci Rep. 2015. PMID: 25801728 Free PMC article. - Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area.
Saint-Mleux B, Eggermann E, Bisetti A, Bayer L, Machard D, Jones BE, Mühlethaler M, Serafin M. Saint-Mleux B, et al. J Neurosci. 2004 Jan 7;24(1):63-7. doi: 10.1523/JNEUROSCI.0232-03.2004. J Neurosci. 2004. PMID: 14715938 Free PMC article.
References
- Alfoldi P, Franken P, Tobler I, Borbely AA. Short light-dark cycles influence sleep stages and EEG power spectra in the rat. Behav Brain Res. 1991;43:125–131. - PubMed
- Benca RM, Bergmann BM, Leung C, Nummy D, Rechtschaffen A. Rat strain differences in response to dark pulse triggering of paradoxical sleep. Physiol Behav. 1991;49:83–87. - PubMed
- Benca RM, Gilliland MA, Obermeyer WH. Effects of lighting conditions on sleep and wakefulness in albino Lewis and pigmented Brown Norway rats. Sleep. 1998;21:451–460. - PubMed
- Crochet S, Sakai K. Effects of microdialysis application of monoamines on the EEG and behavioural states in the cat mesopontine tegmentum. Eur J Neurosci. 1999;11:3738–3752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH12058/MH/NIMH NIH HHS/United States
- NS3397/NS/NINDS NIH HHS/United States
- P50 HL060292/HL/NHLBI NIH HHS/United States
- HL60292/HL/NHLBI NIH HHS/United States
- AG47755/AG/NIA NIH HHS/United States
- F31 MH012058/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources