Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner - PubMed (original) (raw)
Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner
Amy Brewster et al. J Neurosci. 2002.
Abstract
Febrile seizures, in addition to being the most common seizure type of the developing human, may contribute to the generation of subsequent limbic epilepsy. Our previous work has demonstrated that prolonged experimental febrile seizures in the immature rat model increased hippocampal excitability long term, enhancing susceptibility to future seizures. The mechanisms for these profound proepileptogenic changes did not require cell death and were associated with long-term slowed kinetics of the hyperpolarization-activated depolarizing current (I(H)). Here we show that these seizures modulate the expression of genes encoding this current, the hyperpolarization-activated, cyclic nucleotide-gated channels (HCNs): In CA1 neurons expressing multiple HCN isoforms, the seizures induced a coordinated reduction of HCN1 mRNA and enhancement of HCN2 expression, thus altering the neuronal HCN phenotype. The seizure-induced augmentation of HCN2 expression involved CA3 in addition to CA1, whereas for HCN4, mRNA expression was not changed by the seizures in either hippocampal region. This isoform- and region-specific transcriptional regulation of the HCNs required neuronal activity rather than hyperthermia alone, correlated with seizure duration, and favored the formation of slow-kinetics HCN2-encoded channels. In summary, these data demonstrate a novel, activity-dependent transcriptional regulation of HCN molecules by developmental seizures. These changes result in long-lasting alteration of the HCN phenotype of specific hippocampal neuronal populations, with profound consequences on the excitability of the hippocampal network.
Figures
Fig. 1.
Expression patterns of HCN1 and HCN2 immunoreactivity in the hippocampal formation of the immature rat.A–C, HCN1; D–F, HCN2. A, Low-magnification overview of HCN1 protein expression using immunocytochemistry, demonstrating this HCN isoform in the pyramidal cell layers of both CA1 and CA3. B, C, Detail of CA1 and CA3, respectively, demonstrating that the HCN1 isoform is expressed at the protein level in both pyramidal cells [arrows and insets; note the thick cytoplasmic band (boxed areas in A) of signal; see also Fig. 2] and interneurons (arrowheads). Note that in general, immunoreactivity in pyramidal cell somata is weaker than in interneurons, consistent with the distribution of channel proteins to the pyramidal cell dendritic fields (asterisks in A). D, Low-magnification view of HCN2 protein, revealing generally lower expression levels of this isoform. E, F, Detail of the CA1 and CA3 pyramidal cell layer (boxed areas in_D_), respectively, showing expression of the HCN2 isoform in individual eccentric, strongly labeled, presumed interneurons (arrowheads) as well as the much weaker expression in pyramidal cells (arrows and insets). Scale bars: A, D, 600 μm; B, C, E, F, 200 μm; insets, ∼60 μm.
Fig. 2.
Individual hippocampal principal cells and interneurons coexpress HCN1 and HCN2. A–C, Parvalbumin-expressing basket cells in hippocampal CA1 of the immature rat coexpress mRNA for HCN1 (HCN1 mRNA). A, ISH reveals expression of HCN1 mRNA in large neurons within the CA1 pyramidal cell layer (arrow and_arrowhead_). B, Parvalbumin ICC of the same section delineates a parvalbumin-immunoreactive interneuron (arrowhead) as well as the network of axonal boutons innervating parvalbumin-negative pyramidal neurons (arrows). C, The merged image of these confocal microscope sections (see Materials and Methods) demonstrates the coexpression of parvalbumin and HCN1 mRNA in the basket cell (arrowhead) as well as HCN1 expression in parvalbumin-negative presumed pyramidal cells (arrow).D–F, HCN1 protein is coexpressed with HCN2 in individual CA1 pyramidal cell layer neurons. D, ISH demonstrates HCN2 mRNA in the perinuclear cytoplasm of CA1 neurons (arrows). E, ICC demonstrates cytoplasmic HCN1 protein (arrows); the immunoreactivity pattern here, involving broad cytoplasmic patches, can be clearly distinguished from the delicate strands of parvalbumin-immunoreactive interneuronal processes (shown in B). F, The merged image reveals coexpression of both HCN isoforms in individual neurons (arrows), with mRNA generally confined to the perinuclear cytoplasm, versus the peripheral/membranous localization of the channel protein. G–I, Coexpression of HCN1 and HCN2 is also found in CA3. G, HCN2 mRNA; H, HCN1 protein; I, merged image. Scale bar, 20 μm.
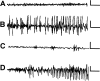
Fig. 3.
Hippocampal recordings of experimental febrile seizures and those generated by kainate. Tracings from bipolar, chronically implanted electrodes in behaving 11-d-old rats demonstrate normal background activity (A). B, The hyperthermia procedure (see Materials and Methods) provoked hippocampal electrographic seizures, manifested as trains of spike waves. C, Preadministration of pentobarbital prevented these hyperthermia-induced seizures. D, Longer hippocampal electrographic seizures were induced by systemic administration of kainate. The evolution of increasing-amplitude spike-wave trains is seen. Calibration: 100 μV, 1 sec.
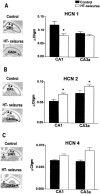
Fig. 4.
Experimental prolonged febrile seizures modulate the expression of HCN subunit isoform in a region- and isoform-specific manner. HCN mRNA levels were quantified after in situ_hybridization analysis (see Materials and Methods). A, Compared with controls, HCN1 mRNA levels of experimental animals (HT-seizures) were significantly (asterisks) reduced in CA1 (arrowheads) but not CA3a. B, Levels of HCN2 mRNA, coding for slower-kinetics channels, were increased by the seizures in both CA1 and CA3 pyramidal cell layers. C, No significant changes in HCN4 mRNA levels were induced by the seizures. The_asterisks in the photomicrograph denote the unique expression of the HCN4 isoform in the medial habenula.
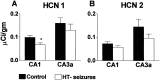
Fig. 5.
The mechanism of HCN mRNA regulation requires seizures rather than hyperthermia and depends on seizure duration. Quantitative analyses of HCN1 and HCN2 mRNAs, to dissect out the contributions of the hyperthermia procedure versus those of the resulting seizures, are shown. A, B, HCN1 expression was reduced by hyperthermia-induced seizures (HT-seizures) in CA1 but not CA3. Blocking the seizures in a group of animals undergoing the same duration and magnitude of hyperthermia [pentobarbital and hyperthermia (PB & HT) group; Table 1] abolished these changes in HCN1. Longer seizures induced by kainate (Table 1) led to more robust modulation of HCN1 expression, also reducing mRNA levels in CA3. C, D, Enhanced HCN2 expression by hyperthermic seizures, found in both CA1 and CA3a pyramidal cell layers, required seizures (i.e., intense neuronal activation). This modulation of HCN expression was not specific to the experimental febrile seizures: prolonged kainate-induced seizures led to the same result. Correlation of HCN expression with seizure duration was found for both HCN1 (A) and HCN2 (C, D) using ANOVA (see Results). *Significantly different from the control group.†SEMs of the control groups in B (0.54%) and D (0.1%) are poorly visualized.
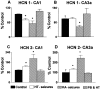
Fig. 6.
Changes in HCN1 expression induced by early life prolonged experimental febrile seizures are long lasting, whereas those in HCN2 are not. HCN mRNA levels were quantified after in situ hybridization analysis (see Materials and Methods) in animals 3 months after the seizures. A, Compared with controls, HCN1 mRNA levels of adult experimental animals (HT-seizures) were significantly (asterisk) reduced in CA1 but not CA3a. This is the same specific pattern found within 1 week of the seizures (Fig.4_A_). Note that the high HCN1 mRNA signal over CA3 reflects interneuronal expression (Santoro et al., 2000; Bender et al., 2001): the resolution of the film autoradiograms does not permit distinguishing interneuronal expression (Brunson et al., 2001).B, Levels of HCN2 mRNA do not differ in CA1 or CA3 of adult control rats compared with those experiencing experimental febrile seizures early in life.
Similar articles
- Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus.
Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Bender RA, et al. J Neurosci. 2003 Jul 30;23(17):6826-36. doi: 10.1523/JNEUROSCI.23-17-06826.2003. J Neurosci. 2003. PMID: 12890777 Free PMC article. - Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus.
Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Brewster AL, et al. Cereb Cortex. 2007 Mar;17(3):702-12. doi: 10.1093/cercor/bhk021. Epub 2006 Apr 28. Cereb Cortex. 2007. PMID: 16648453 Free PMC article. - Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1-4 suggests evolving roles in the developing rat hippocampus.
Bender RA, Brewster A, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ. Bender RA, et al. Neuroscience. 2001;106(4):689-98. doi: 10.1016/s0306-4522(01)00314-1. Neuroscience. 2001. PMID: 11682156 Free PMC article. - Febrile seizures: mechanisms and relationship to epilepsy.
Dubé CM, Brewster AL, Baram TZ. Dubé CM, et al. Brain Dev. 2009 May;31(5):366-71. doi: 10.1016/j.braindev.2008.11.010. Epub 2009 Feb 15. Brain Dev. 2009. PMID: 19232478 Free PMC article. Review. - HCN channelopathies: pathophysiology in genetic epilepsy and therapeutic implications.
Reid CA, Phillips AM, Petrou S. Reid CA, et al. Br J Pharmacol. 2012 Jan;165(1):49-56. doi: 10.1111/j.1476-5381.2011.01507.x. Br J Pharmacol. 2012. PMID: 21615728 Free PMC article. Review.
Cited by
- Sub region-specific modulation of synchronous neuronal burst firing after a kainic acid insult in organotypic hippocampal cultures.
Reid CA, Adams BE, Myers D, O'Brien TJ, Williams DA. Reid CA, et al. BMC Neurosci. 2008 Jul 2;9:59. doi: 10.1186/1471-2202-9-59. BMC Neurosci. 2008. PMID: 18593482 Free PMC article. - Ion Channels in Genetic Epilepsy: From Genes and Mechanisms to Disease-Targeted Therapies.
Oyrer J, Maljevic S, Scheffer IE, Berkovic SF, Petrou S, Reid CA. Oyrer J, et al. Pharmacol Rev. 2018 Jan;70(1):142-173. doi: 10.1124/pr.117.014456. Pharmacol Rev. 2018. PMID: 29263209 Free PMC article. Review. - Early treatment suppresses the development of spike-wave epilepsy in a rat model.
Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Blumenfeld H, et al. Epilepsia. 2008 Mar;49(3):400-9. doi: 10.1111/j.1528-1167.2007.01458.x. Epub 2007 Dec 6. Epilepsia. 2008. PMID: 18070091 Free PMC article. - TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain.
Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA. Santoro B, et al. Neuron. 2009 Jun 25;62(6):802-13. doi: 10.1016/j.neuron.2009.05.009. Neuron. 2009. PMID: 19555649 Free PMC article. - HCN channels in behavior and neurological disease: too hyper or not active enough?
Lewis AS, Chetkovich DM. Lewis AS, et al. Mol Cell Neurosci. 2011 Feb;46(2):357-67. doi: 10.1016/j.mcn.2010.11.007. Epub 2010 Dec 3. Mol Cell Neurosci. 2011. PMID: 21130878 Free PMC article. Review.
References
- Bender RA, Baram TZ. Do prolonged febrile seizures injure hippocampal neurons? Insights from animal models. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic; San Diego: 2002. pp. 127–137.
- Bender RA, Dubé C, Baram TZ. Mossy fiber sprouting into the inner molecular layer of the dentate gyrus follows prolonged febrile seizures in the immature rat model. Epilepsia. 2000;41 [Suppl 7]:76.
- Bender RA, Brewster A, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ. Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1–4 suggests evolving roles in the developing rat hippocampus. Neuroscience. 2001;106:689–698. - PMC - PubMed
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS28912-S1/NS/NINDS NIH HHS/United States
- R01 NS035439/NS/NINDS NIH HHS/United States
- R37 NS035439/NS/NINDS NIH HHS/United States
- NS 35439/NS/NINDS NIH HHS/United States
- NS 28912/NS/NINDS NIH HHS/United States
- R01 NS028912/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous