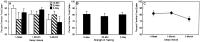Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum - PubMed (original) (raw)
Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum
Robert E Clark et al. J Neurosci. 2002.
Abstract
We studied the importance of the hippocampus and subiculum for anterograde and retrograde memory in the rat using social transmission of food preference, a nonspatial memory task. Experiment 1 asked how long an acquired food preference could be remembered. In experiment 2, we determined the anterograde amnesic effects of large lesions of the hippocampus that included the subiculum. In experiment 3, large lesions of the hippocampus that included the subiculum were made 1, 10, or 30 d after learning to determine the nature and extent of retrograde amnesia. Normal rats exhibited memory of the acquired food preference for at least 3 months after learning. Hippocampal lesions that included the subiculum produced marked anterograde amnesia and a 1-30 d temporally graded retrograde amnesia. The results show the importance of the hippocampus and related structures for nonspatial memory and also demonstrate the temporary role of these structures in long-term memory.
Figures
Fig. 1.
A, The influence of strength of training on percent preference for the familiar food across delays.White bars indicate performance of groups given a 10 min interaction with demonstrator rats, _striped bars_indicate performance of groups given a 30 min interaction with demonstrator rats, and black bars indicate performance of groups given 30 min interactions with different demonstrator rats on 3 consecutive days. B, The influence of strength of training when the delays were combined. C, Percentage of preference for the familiar food across delays (strength of training combined at each delay). Error bars show SEM.
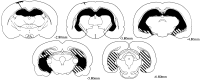
Fig. 2.
Reconstructions of coronal sections showing the largest (striped) and smallest (black) lesions in rats with ibotenic acid lesions. Each series of sections progresses (left to right) from anterior to posterior levels. Numbers represent the distance in millimeters posterior to bregma.
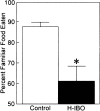
Fig. 3.
Percent preference for the familiar food for control (n = 16) animals and H-IBO (n = 15) animals. Training occurred after the lesions were made, and retention was tested 48 hr later. The control group performed above chance and scored higher than the H-IBO group. The H-IBO group did not perform above chance. *p < 0.05, significant difference between the two groups. Error bars show SEM.
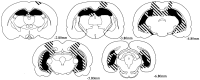
Fig. 4.
Reconstructions of coronal sections showing the largest (striped) and smallest (black) lesion in rats with radiofrequency lesions. Each series of sections progresses (left to right) from anterior to posterior levels. Numbers represent the distance in millimeters posterior to bregma.
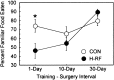
Fig. 5.
Percent preference for the familiar food for control animals (CON) and H-RF animals across three training–surgical intervals. For the 1 d condition, the control group performed significantly better than the H-RF group. The H-RF groups did not perform above chance in the 1 and 10 d conditions. In the 30 d condition, the two groups performed similarly, and both groups performed well above chance. *p < 0.05, significant difference between the control and H-RF groups. Error bars show SEM.
Similar articles
- Anterograde and retrograde amnesia in rats with large hippocampal lesions.
Winocur G, McDonald RM, Moscovitch M. Winocur G, et al. Hippocampus. 2001;11(1):18-26. doi: 10.1002/1098-1063(2001)11:1<18::AID-HIPO1016>3.0.CO;2-5. Hippocampus. 2001. PMID: 11261769 - Selective lesions of basal forebrain cholinergic neurons produce anterograde and retrograde deficits in a social transmission of food preference task in rats.
Vale-Martínez A, Baxter MG, Eichenbaum H. Vale-Martínez A, et al. Eur J Neurosci. 2002 Sep;16(6):983-98. doi: 10.1046/j.1460-9568.2002.02153.x. Eur J Neurosci. 2002. PMID: 12383228 - Retrosplenial cortex damage produces retrograde and anterograde context amnesia using strong fear conditioning procedures.
Fournier DI, Eddy MC, DeAngeli NE, Huszár R, Bucci DJ. Fournier DI, et al. Behav Brain Res. 2019 Sep 2;369:111920. doi: 10.1016/j.bbr.2019.111920. Epub 2019 Apr 27. Behav Brain Res. 2019. PMID: 31039379 Free PMC article. - Amnesia and the hippocampus.
Cipolotti L, Bird CM. Cipolotti L, et al. Curr Opin Neurol. 2006 Dec;19(6):593-8. doi: 10.1097/01.wco.0000247608.42320.f9. Curr Opin Neurol. 2006. PMID: 17102699 Review. - Hippocampus and retrograde amnesia in the rat model: a modest proposal for the situation of systems consolidation.
Sutherland RJ, Sparks FT, Lehmann H. Sutherland RJ, et al. Neuropsychologia. 2010 Jul;48(8):2357-69. doi: 10.1016/j.neuropsychologia.2010.04.015. Epub 2010 Apr 27. Neuropsychologia. 2010. PMID: 20430043 Free PMC article. Review.
Cited by
- Synaptic rearrangement of NMDA receptors controls memory engram formation and malleability in the cortex.
Bessières B, Dupuis J, Groc L, Bontempi B, Nicole O. Bessières B, et al. Sci Adv. 2024 Aug 30;10(35):eado1148. doi: 10.1126/sciadv.ado1148. Epub 2024 Aug 30. Sci Adv. 2024. PMID: 39213354 Free PMC article. - Cannabidiol enhances socially transmitted food preference: a role of acetylcholine in the mouse basal forebrain.
Chang CY, Dai W, Hu SS. Chang CY, et al. Psychopharmacology (Berl). 2024 Aug 19. doi: 10.1007/s00213-024-06670-1. Online ahead of print. Psychopharmacology (Berl). 2024. PMID: 39158618 - A cognitive map for object memory in the hippocampus.
Manns JR, Eichenbaum H. Manns JR, et al. Learn Mem. 2009 Sep 30;16(10):616-24. doi: 10.1101/lm.1484509. Print 2009 Oct. Learn Mem. 2009. PMID: 19794187 Free PMC article. - Human brain activity and functional connectivity as memories age from one hour to one month.
Tallman CW, Clark RE, Smith CN. Tallman CW, et al. Cogn Neurosci. 2022 Jul;13(3-4):115-133. doi: 10.1080/17588928.2021.2021164. Epub 2022 Jan 24. Cogn Neurosci. 2022. PMID: 35073239 Free PMC article. - Acetylcholine release in the hippocampus and prelimbic cortex during acquisition of a socially transmitted food preference.
Gold PE, Countryman RA, Dukala D, Chang Q. Gold PE, et al. Neurobiol Learn Mem. 2011 Oct;96(3):498-503. doi: 10.1016/j.nlm.2011.08.004. Epub 2011 Aug 30. Neurobiol Learn Mem. 2011. PMID: 21907814 Free PMC article.
References
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. - PubMed
- Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG05131/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- MH 24600/MH/NIMH NIH HHS/United States
- R37 MH024600/MH/NIMH NIH HHS/United States
- R01 MH024600/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical