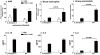Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma - PubMed (original) (raw)
. 2002 Jun 3;195(11):1433-44.
doi: 10.1084/jem.20020009.
Ming Yang, Surendran Mahalingam, Joachim Kuehr, Dianne C Webb, Ljubov Simson, Simon P Hogan, Aulikki Koskinen, Andrew N J McKenzie, Lindsay A Dent, Marc E Rothenberg, Klaus I Matthaei, Ian G Young, Paul S Foster
Affiliations
- PMID: 12045241
- PMCID: PMC2193548
- DOI: 10.1084/jem.20020009
Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma
Joerg Mattes et al. J Exp Med. 2002.
Abstract
Interleukin (IL)-5 and IL-13 are thought to play key roles in the pathogenesis of asthma. Although both cytokines use eotaxin to regulate eosinophilia, IL-13 is thought to operate a separate pathway to IL-5 to induce airways hyperreactivity (AHR) in the allergic lung. However, identification of the key pathway(s) used by IL-5 and IL-13 in the disease process is confounded by the failure of anti-IL-5 or anti-IL-13 treatments to completely inhibit the accumulation of eosinophils in lung tissue. By using mice deficient in both IL-5 and eotaxin (IL-5/eotaxin(-/-)) we have abolished tissue eosinophilia and the induction of AHR in the allergic lung. Notably, in mice deficient in IL-5/eotaxin the ability of CD4(+) T helper cell (Th)2 lymphocytes to produce IL-13, a critical regulator of airways smooth muscle constriction and obstruction, was significantly impaired. Moreover, the transfer of eosinophils to IL-5/eotaxin(-/-) mice overcame the intrinsic defect in T cell IL-13 production. Thus, factors produced by eosinophils may either directly or indirectly modulate the production of IL-13 during Th2 cell development. Our data show that IL-5 and eotaxin intrinsically modulate IL-13 production from Th2 cells and that these signaling systems are not necessarily independent effector pathways and may also be integrated to regulate aspects of allergic disease.
Figures
Figure 1.
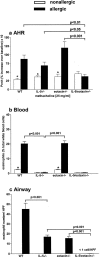
IL-5 and eotaxin deficiency abrogates AHR. Groups of mice (WT, IL-5 −/−, eotaxin−/−, or IL-5/eotaxin−/−) were sensitized with saline (nonallergic) or OVA (allergic) and aeroallergen challenged with OVA. (a) AHR data represent the percentage increase in Penh at 25 mg/ml methacholine over baseline reactivity in the absence of cholinergic stimuli. The maximal response to methacholine (25 mg/ml) is shown, but these results are representative of the full dose response curve. Data is the mean of six to eight mice ± SEM per group. (b) The percentages of blood eosinophils. Data is the mean ± SEM of four mice per group. (c) Mean number of eosinophils residing in the airway wall per 10 similar HPFs (×1,000) for each group. Data is the mean ± SEM of four mice per group). *, significant differences (P < 0.05) between respective nonallergic and allergic groups. Levels of significant differences are also indicated for other groups.
Figure 2.
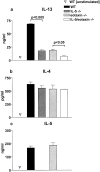
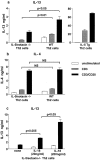
Intrinsic defect in IL-13 production by antigen-specific CD4+ T cells in the absence of IL-5 and eotaxin. Production of (a) IL-13, (b) IL-4, and (c) IL-5 by purified OVA-specific CD4+ T cells generated from WT, IL-5 −/−, eotaxin−/−, or IL-5/eotaxin−/− mice. Cultures were performed in triplicate. CD4+ T cells were derived from splenocytes because these T cell cytokine profiles were directly reflective of those observed after the stimulation of PBLN cultures. Data represent the mean ± SEM of n = 4 cultures per group. Significant differences (P < 0.05) between respective groups are shown.
Figure 3.
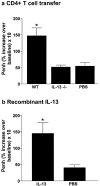
Adoptive transfer of IL-13–producing CD4+ T cells to naive IL-5/eotaxin−/− mice reconstitutes normal IL-13 levels in the allergic lung and induces AHR and eosinophilia. CD4+ T cells (2 × 106 per mouse) were adoptively transferred into IL-5/eotaxin−/− or WT mice. Control groups received PBS. (a) AHR. Data represent the percentage increase in Penh at 25 mg/ml methacholine over baseline reactivity in the absence of cholinergic stimuli. The maximal response to methacholine (25 mg/ml) is shown, but these results are representative of the full dose response curve. Data is the mean of six to eight mice ± SEM per group. (b) The percentage of blood eosinophils. Data represent the mean ± SEM of three or four mice per group. (c) Mean number of airway wall/smooth muscle eosinophils per 10 similar HPF (×1,000) for each group. Data mean ± SEM of two or three mice per group. (d–f) Production of (d) IL-13, (e) IL-4, and (f) IL-5 from PBLN cells after OVA restimulation. T cell cytokine levels were determined in PBLN culture because these directly reflect the trafficking of the transferred cells to the lung and the subsequent release of cytokines in the pulmonary compartment. *, significant differences (P < 0.05) between control and T cell transferred groups. Levels of significant differences are also indicated for other groups.
Figure 4.
IL-13 plays a critical role in the generation of AHR. (a) Adoptive transfer of IL-13–deficient CD4+ T cells (2 × 106 per mouse) to naive IL-5/eotaxin−/− mice failed to induce AHR. Control groups received PBS. (b) In contrast, instillation of mIL-13 in the lungs of naive IL-5/eotaxin−/− mice induced AHR. Data is the mean of four to eight mice ± SEM per group. *, significant differences (P < 0.05) between groups is shown.
Figure 5.
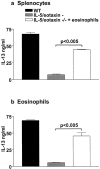
Adoptive transfer of eosinophils to IL-5/eotaxin−/− mice reestablishes normal production of IL-13 from antigen-specific CD4+ T cells. IL-13 production from purified CD4+ T cells derived from allergic WT and IL-5/eotaxin−/− mice or allergic IL-5/eotaxin−/− mice that had been injected with eosinophils intraperitoneally before sensitization. CD4+ T cells stimulated in the presence of OVA with (a) freshly isolated and mitomycin C–treated splenocytes or (b) with purified eosinophils. Cultures were performed in triplicate. Data represent the mean ± SEM of n = 4 cultures per group. Significant differences (P < 0.05) between respective groups are shown. Cytokine production was analyzed from T cells derived from splenocytes after eosinophil transfer experiments. Eosinophils injected intraperitoneally migrate to the spleen and colocalize with T cells in this compartment. Thus, by taking T cells from the spleen we know that they have been exposed to the transferred eosinophil population.
Figure 6.
IL-18 expression is decreased in PBLN of allergic IL-5/eotaxin−/− mice and up-regulated in IL-5–primed eosinophils. (a) PBLN cells from WT, IL-5−/−, and IL-5/eotaxin−/− were isolated and RT-PCR was performed for HPRT, IL-5, eotaxin, IL-13, and IL-18. To confirm specificity, PCR products were probed by Southern blot analysis using 30-mer oligonucleotide probes for these factors. The mean fold decreases in cytokine levels in comparison to WT are as follows: eotaxin in IL-5−/− (10-fold reduction); IL-13 in IL-5−/− (15-fold reduction) and in IL-5/eotaxin−/− (40-fold reduction); and IL-18 in IL-5−/− (15-fold reduction) and IL-5/eotaxin−/− (30-fold reduction). PBLN were analyzed to directly reflect the production of factors within the allergic lung. (b) Highly purified eosinophils were stimulated with IL-5/anti-CD28 or IgA–anti-IgA complex for 18 h to activate cytokine gene expression. RT-PCR was performed for HPRT, CCR3, IL-18, IL-13, IL-4, and IL-12 (p40). PCR products were probed by Southern blot analysis using 30-mer oligonucleotide probes for these factors.
Figure 7.
IL-13 production is impaired in polarized Th2 cells derived from IL-5/eotaxin−/− mice, but the defect is overcome by addition of IL-18 to cultures. Production of (a) IL-13 and (b) IL-4 by CD4+ Th2 cells after polyclonal stimulation. Th2 cells were generated from purified naive CD4+ T cells isolated from the spleens of WT, IL-5/eotaxin−/−, or IL-5 Tg mice. (c) Release of IL-13 by CD4+ Th2 cells after polyclonal (solid bar) in the absence or presence of IL-18 (20 and 200 ng/ml, respectively). Cultures were performed in triplicate. Data represent the mean ± SEM of n = 4 cultures per group. The spleen was used as a source of naive T cells to enable the generation of sufficient Th2 cells.
Similar articles
- The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity.
Mould AW, Ramsay AJ, Matthaei KI, Young IG, Rothenberg ME, Foster PS. Mould AW, et al. J Immunol. 2000 Feb 15;164(4):2142-50. doi: 10.4049/jimmunol.164.4.2142. J Immunol. 2000. PMID: 10657668 - IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung.
Mattes J, Yang M, Siqueira A, Clark K, MacKenzie J, McKenzie AN, Webb DC, Matthaei KI, Foster PS. Mattes J, et al. J Immunol. 2001 Aug 1;167(3):1683-92. doi: 10.4049/jimmunol.167.3.1683. J Immunol. 2001. PMID: 11466392 - Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin.
Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. Yang M, et al. Am J Respir Cell Mol Biol. 2001 Oct;25(4):522-30. doi: 10.1165/ajrcmb.25.4.4620. Am J Respir Cell Mol Biol. 2001. PMID: 11694459 - Elemental signals regulating eosinophil accumulation in the lung.
Foster PS, Mould AW, Yang M, Mackenzie J, Mattes J, Hogan SP, Mahalingam S, Mckenzie AN, Rothenberg ME, Young IG, Matthaei KI, Webb DC. Foster PS, et al. Immunol Rev. 2001 Feb;179:173-81. doi: 10.1034/j.1600-065x.2001.790117.x. Immunol Rev. 2001. PMID: 11292021 Review. - Distinct spatial requirement for eosinophil-induced airways hyperreactivity.
Webb DC, McKenzie AN, Matthaei KI, Rothenberg ME, Foster PS. Webb DC, et al. Immunol Cell Biol. 2001 Apr;79(2):165-9. doi: 10.1046/j.1440-1711.2001.00989.x. Immunol Cell Biol. 2001. PMID: 11264712 Review.
Cited by
- Vinpocetine alleviates lung inflammation via macrophage inflammatory protein-1β inhibition in an ovalbumin-induced allergic asthma model.
Choi WS, Kang HS, Kim HJ, Lee WT, Sohn UD, Lee JY. Choi WS, et al. PLoS One. 2021 Apr 29;16(4):e0251012. doi: 10.1371/journal.pone.0251012. eCollection 2021. PLoS One. 2021. PMID: 33914833 Free PMC article. - Until Death Do Us Part: Necrosis and Oxidation Promote the Tumor Microenvironment.
Lotfi R, Kaltenmeier C, Lotze MT, Bergmann C. Lotfi R, et al. Transfus Med Hemother. 2016 Mar;43(2):120-32. doi: 10.1159/000444941. Epub 2016 Mar 8. Transfus Med Hemother. 2016. PMID: 27226794 Free PMC article. Review. - CCL7 and IRF-7 Mediate Hallmark Inflammatory and IFN Responses following Rhinovirus 1B Infection.
Girkin J, Hatchwell L, Foster P, Johnston SL, Bartlett N, Collison A, Mattes J. Girkin J, et al. J Immunol. 2015 May 15;194(10):4924-30. doi: 10.4049/jimmunol.1401362. Epub 2015 Apr 6. J Immunol. 2015. PMID: 25847975 Free PMC article. - Eosinophils enhance WNT-5a and TGF-β1 genes expression in airway smooth muscle cells and promote their proliferation by increased extracellular matrix proteins production in asthma.
Januskevicius A, Vaitkiene S, Gosens R, Janulaityte I, Hoppenot D, Sakalauskas R, Malakauskas K. Januskevicius A, et al. BMC Pulm Med. 2016 Jun 13;16(1):94. doi: 10.1186/s12890-016-0254-9. BMC Pulm Med. 2016. PMID: 27297409 Free PMC article. Clinical Trial. - Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma.
Barthel SR, Johansson MW, McNamee DM, Mosher DF. Barthel SR, et al. J Leukoc Biol. 2008 Jan;83(1):1-12. doi: 10.1189/jlb.0607344. Epub 2007 Oct 10. J Leukoc Biol. 2008. PMID: 17906117 Free PMC article. Review.
References
- Bochner, B.S., B.J. Undem, and L.M. Lichtenstein. 1994. Immunological aspects of allergic asthma. Annu. Rev. Immunol. 12:295–335. - PubMed
- Wills-Karp, M. 1999. Immunological basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255–281. - PubMed
- Gleich, G.J., and C. Adolphson. 1993. Bronchial hyperreactivity and eosinophil granule proteins. Agents Actions Suppl. 43:223–230. - PubMed
- Azzawi, M., B. Bradley, P.K. Jeffery, A.J. Frew, A.J. Wardlaw, G. Knowles, B. Assoufi, J.V. Collins, S. Durham, and A.B. Kay. 1990. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am. Rev. Respir. Dis. 142:1407–1413. - PubMed
- Bentley, A.M., Q. Meng, D.S. Robinson, Q. Hamid, A.B. Kay, and S.R. Durham. 1993. Increases in activated T lymphocytes, eosinophils, and cytokine mRNA expression for interleukin-5 and granulocyte/macrophage colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopic asthmatics. Am. J. Respir. Cell Mol. Biol. 8:35–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials