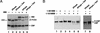Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex - PubMed (original) (raw)
Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex
Christy J Fryer et al. Genes Dev. 2002.
Abstract
Signaling through the Notch pathway activates the proteolytic release of the Notch intracellular domain (ICD), a dedicated transcriptional coactivator of CSL enhancer-binding proteins. Here we show that chromatin-dependent transactivation by the recombinant Notch ICD-CBF1 enhancer complex in vitro requires an additional coactivator, Mastermind (MAM). MAM provides two activation domains necessary for Notch signaling in mammalian cells and in Xenopus embryos. We show that the central MAM activation domain (TAD1) recruits CBP/p300 to promote nucleosome acetylation at Notch enhancers and activate transcription in vitro. We also find that MAM expression induces phosphorylation and relocalization of endogenous CBP/p300 proteins to nuclear foci in vivo. Moreover, we show that coexpression with MAM and CBF1 strongly enhances phosphorylation and proteolytic turnover of the Notch ICD in vivo. Enhanced phosphorylation of the ICD and p300 requires a glutamine-rich region of MAM (TAD2) that is essential for Notch transcription in vivo. Thus MAM may function as a timer to couple transcription activation with disassembly of the Notch enhancer complex on chromatin.
Figures
Figure 1
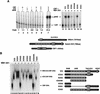
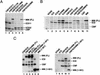
MAM interacts with and is required for chromatin-dependent transcriptional activation by the Notch (CBF1:ICD) enhancer complex in vitro. (A) Primer-extension analysis of the transcriptional activity of purified recombinant Notch complexes on pNRE chromatin in vitro. Where indicated, the reactions either lacked enhancer factors (lanes 1,8), or contained CBF1 (120 nM, lanes _2,5_–_7,9_–14), the wild-type Xenopus Notch ICD (120 nM, lanes 3,6,7,9), or different mutant ICD proteins (lanes 10_–_14), and human 1–301MM (120 nM, lanes 4,5,7,9_–_14). Arrows indicate transcripts originating from the pNRE or control (nonchromatin) α-globin (α-glo) templates. The Notch enhancer complex is assembled through binding of the ICD ankyrin repeat (ANK) domain to the central region of CBF1 (amino acids 179–361) and to an N-terminal domain of MAM, as indicated schematically at the bottom of the figure. (B) EMSA analysis of the ability of wild-type and mutant ICD proteins (lanes 3_–_18, as indicated above each lane) to support a stable ternary complex with CBF1 (lanes 2_–_18) and MAM (1–301MM; lanes 3,5,7,9,11,13,15) on DNA in vitro. Arrows indicate the various CBF1, ICD–CBF1, and MAM:ICD–CBF1 complexes on DNA. The different mutant ICD proteins tested are depicted to the right of the figure.
Figure 2
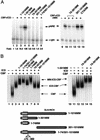
MAM activates pNRE transcription on chromatin in vitro through an N-terminal activation domain (TAD1). (A, left panel) Analysis of the ability of various MAM proteins to activate pNRE transcription in the presence of CBF1 and ICD in vitro. The different MAM truncation or deletion mutants tested are indicated above each lane and are shown schematically at the bottom of the figure. Transcription conditions are as described for Figure 1A. (Right panel) The isolated MAM TAD1 fragment (amino acids 75–301) selectively blocks Notch transcription in vitro. Reactions either lacked the Notch enhancer complex (lane 9) or contained the complex (120nM MAM:ICD–CBF1; lanes 10_–_14) in the absence (lane 10) or presence of 75–301MM (480 nM, lane 11; 960 nM, lane 12), a C-terminal fragment of MAM (amino acids 301–1016 fragment, 480 nM; lane 13), or the β-catenin CTARM transactivation domain (960 nM, lane 14). (B, left panel) EMSA analysis of Notch complexes containing wild-type or mutant MAM proteins, as indicated above each lane. Reactions either lacked enhancer factors (lane 1) or contained CBF1 (lanes 2_–_9), the ICD (lanes 3_–_9), and various MAM proteins (lanes 4_–_9), as indicated above each lane. (Right panel) The MAM TAD1 fragment (amino acids 75–301) does not disrupt assembly of the Notch enhancer complex on DNA. The binding reactions either lacked enhancer factors (lane 10) or contained 100 nM each of CBF1 (lanes 11_–_15), the ICD (lanes 12_–_15), and 1–1016MM (lanes 14,15), either in the absence (lanes 12,14) or presence (lanes 13,15) of a fivefold excess of 75–301MM. Arrows indicate the different Notch enhancer factor:DNA complexes.
Figure 3
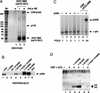
MAM interacts specifically with nuclear CBP/p300 and promotes p300-mediated nucleosome acetylation in vitro. (A) SDS-PAGE analysis of HeLa nuclear proteins that interact with the MAM TAD1 fragment. A crude HeLa nuclear extract was incubated with either glutathione beads (lane 1) or GST–MAMTAD1 beads (lane 3), and the associated proteins were eluted by boiling and analyzed by SDS-PAGE and silver staining. The arrow indicates a band identified as CBP/p300 through tryptic digestion and MALDI-TOF mass-spectrometry analysis. (B) HeLa nuclear extract was incubated with either GST (lane 8) or various GST–MAM protein-coupled beads (lanes 2_–_7) as indicated above each lane, and analyzed by immunoblotting for associated nuclear p300 protein. The input nuclear p300 protein is shown in lane 1. (C) Purified recombinant p300 enhances Notch transcription in vitro. Transcription reactions contained the pNRE template incubated in the absence of enhancer factors (lane 1,5) or with the Notch enhancer complex (MAM–ICD–CBF1; lanes 6,7), which was added following chromatin assembly. Where indicated, recombinant p300 was incubated with the chromatin template for 30 min prior to analysis of transcription by primer extension (lanes 2_–_4,7). (D) Purified p300 promotes acetylation of pNRE nucleosomal histones in a MAM-dependent manner in vitro. The pNRE chromatin assembly reactions either lacked enhancer factors (lane 1), or contained CBF1–ICD (lane 2_–_4,6) and wild-type or mutant MAM protein (lanes 3_–_6), as indicated above the lanes. The pNRE template was purified following chromatin assembly and incubated with recombinant p300 and 14C-acetyl CoA, and the labeled nucleosomal histones were identified by SDS-PAGE and fluorography.
Figure 4
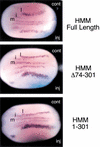
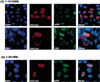
Both TAD1 and the C-terminal activation domain (TAD2) of MAM are required for Notch signaling in injected Xenopus embryos. Xenopus embryos at the two-cell stage were injected with RNA encoding wild-type (Full-length) or mutant human MAM proteins (HMMΔ74–301; HMM1–301) along with lacZ RNA as a tracer. The embryos were fixed and stained for β-galactosidase expression and double-labeled by whole-mount in situ hybridization for a neural-specific tubulin. Notch signaling was assessed by counting the number of primary neurons on the neural plate for the half of the embryo that was injected (inj) relative to that for the uninjected control (cont). (m) Midline; (i) interneuron; (l) lateral neuron.
Figure 5
Human MAM directs endogeneous CBP/p300 to nuclear foci in vivo in a TAD2-dependent manner. Transiently expressed Myc-tagged wild-type (1–1016MM) or mutant MAM proteins (1–301MM) and endogenous p300 and CBP proteins were visualized by indirect immunofluorescence and deconvolution microscopy in HeLa cells. (A) HeLa cells transiently transfected with 1016MM-Myc were imaged for Myc immunofluorescence (red) and endogenous p300 (upper panel) or CBP (lower panel) immunofluorescence (green). Cells were counterstained with DAPI (blue) to show the position of the nucleus for all the cells in the field. (B) HeLa cells were transiently transfected with 1–301MM-Myc and analyzed as in A.
Figure 6
The MAM TAD2 region promotes the modification and turnover of Notch ICD in vivo. (A) MAM and CBF1 enhance the PEST-dependent proteolytic turnover of the Notch ICD in vivo. The expression of Myc-tagged Notch enhancer proteins in C2C12 cells was detected 48 h after transfection by SDS-PAGE of cell extracts and immunoblotting with an anti-Myc antibody. The expressed proteins included CBF1 (lanes 1_–_6), full-length ICD (ICD11; lanes 1_–_3), a C-terminal truncated ICD lacking the PEST domain (ICD22, lanes 4_–_6), full-length MAM (1–1016MM; lanes 3_–_6), and the Notch-specific factor NRARP (N, lanes 2,5). (B) MAM-dependent turnover of the Notch ICD requires CBF1 in vivo. Full-length Notch ICD (ICD; lanes 2_–_9), MAM (MM; lanes 4,5,8,9,11), CBF1 (lanes 6_–_10), and NRARP (lanes 3,5,7,9) were expressed in HeLa cells and analyzed for protein expression 48 h after transfection in vivo. (C) HeLa cells (left panel) or C2C12 cells (right panel) were transfected with CBF1 (lanes 1,2,5,9_–_11), ICD (lanes 1,2,4,5,8_–_11), and either full-length (FL; lanes 2_–_4,10) or 1–301MM (1–301; lanes 5,6,11), and protein levels were examined as in A. The MAM-dependent modification of the ICD is evidenced by its altered mobility by SDS-PAGE (lane 10).
Figure 7
Transient expression of MAM alters the phosphorylation of the Notch ICD and endogenous CBP proteins. (A) C2C12 cells were transfected with myc-tagged forms of ICD and CBF1 in the absence (lanes 1_–_3) or presence (lanes 4_–_6) of MAM, and cell extracts prepared 48 h after transfection were immunoprecipitated with an anti-myc antibody (9E10). The isolated proteins were resuspended in phosphatase buffer (lanes 1,4), phosphatase buffer containing 0.2 U of acid phosphatase (lanes 2,5), or phosphatase buffer containing 0.2 U of acid phosphatase plus 1 mM sodium orthovanadate (Van; lanes 3,6). Immunoprecipitated complexes were detected by Western blotting with an anti-myc antibody. (B) 293T cells were transfected with pCDNA empty vector (lane 1), 1–301MM (lane 2), or 1–1016MM (lanes 3_–_6), and cell extracts prepared 48 h after transfection were immunoprecipitated with an anti-CBP antibody. The isolated complexes were untreated (lanes 1_–_3) or resuspended in phosphatase buffer (lane 4), phosphatase buffer containing 0.2 U of acid phosphatase (lane 5), or phosphatase buffer containing 0.2 U of acid phosphatase plus 1 mM sodium orthovanadate (Van; lane 6). The immunoprecipitated complexes were detected by Western blotting with an anti-CBP antibody. Lanes 7 and 8 show a longer exposure of the reactions shown in lanes 5 and 6.
Figure 8
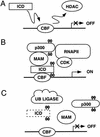
Model for the mechanism of Notch transcription. (A) Notch target genes are repressed through CBF1 complexes that contain histone deacetylases (HDAC) and other corepressors (Dou et al. 1994; Kao et al. 1998; Hsieh et al. 1999; Morel et al. 2001). Although previous studies have shown that binding of the Notch ICD to CBF1 can displace corepressor complexes (Kao et al. 1998; Zhou et al. 2000), the data presented here indicate that a CBF1–ICD complex would be insufficient to activate Notch transcription in the absence of MAM. (B) A three-way complex containing CBF1, ICD, and MAM is required for Notch transcription on chromatin templates in vitro. CBP/p300 is recruited through the MAM TAD1 region to promote transcription initiation and acetylate nearby nucleosomal histones. The ICD activation domain also contributes to transcription at this step. We find that MAM induces the phosphorylation of both CBP/p300 and the Notch ICD in a step that requires the MAM TAD2 region. Phosphorylation of the Notch ICD by MAM also requires CBF1, which may stabilize binding of MAM to the ICD (Petcherski and Kimble 2000b). Widespread phosphorylation of CBP/p300 proteins by MAM may contribute to the accumulation of these proteins in nuclear foci. It remains to be determined whether the MAM-induced phosphorylation events might be carried out by cyclin-dependent kinases associated with the RNA polymerase II (RNAPII) complex (Price 2000; Orphanides and Reinberg 2002) or other protein kinases associated with MAM. (C) The phosphorylated ICD may be targeted for ubiquitination by ubiquitin ligase complexes, leading to disassembly of the enhancer complex and proteolytic degradation of the ICD.
Similar articles
- Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover.
Fryer CJ, White JB, Jones KA. Fryer CJ, et al. Mol Cell. 2004 Nov 19;16(4):509-20. doi: 10.1016/j.molcel.2004.10.014. Mol Cell. 2004. PMID: 15546612 - A human protein with sequence similarity to Drosophila mastermind coordinates the nuclear form of notch and a CSL protein to build a transcriptional activator complex on target promoters.
Kitagawa M, Oyama T, Kawashima T, Yedvobnick B, Kumar A, Matsuno K, Harigaya K. Kitagawa M, et al. Mol Cell Biol. 2001 Jul;21(13):4337-46. doi: 10.1128/MCB.21.13.4337-4346.2001. Mol Cell Biol. 2001. PMID: 11390662 Free PMC article. - p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by notch intracellular domains in vitro.
Wallberg AE, Pedersen K, Lendahl U, Roeder RG. Wallberg AE, et al. Mol Cell Biol. 2002 Nov;22(22):7812-9. doi: 10.1128/MCB.22.22.7812-7819.2002. Mol Cell Biol. 2002. PMID: 12391150 Free PMC article. - Structures of CSL, Notch and Mastermind proteins: piecing together an active transcription complex.
Kovall RA. Kovall RA. Curr Opin Struct Biol. 2007 Feb;17(1):117-27. doi: 10.1016/j.sbi.2006.11.004. Epub 2006 Dec 6. Curr Opin Struct Biol. 2007. PMID: 17157496 Review. - Notch pathway: making sense of suppressor of hairless.
Bray S, Furriols M. Bray S, et al. Curr Biol. 2001 Mar 20;11(6):R217-21. doi: 10.1016/s0960-9822(01)00109-9. Curr Biol. 2001. PMID: 11301266 Review.
Cited by
- NOTCH1 mediates a switch between two distinct secretomes during senescence.
Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, Antrobus R, Tomimatsu K, Howat W, Lehner PJ, Zender L, Narita M. Hoare M, et al. Nat Cell Biol. 2016 Sep;18(9):979-92. doi: 10.1038/ncb3397. Epub 2016 Aug 15. Nat Cell Biol. 2016. PMID: 27525720 Free PMC article. - Notch3 and the Notch3-upregulated RNA-binding protein HuD regulate Ikaros alternative splicing.
Bellavia D, Mecarozzi M, Campese AF, Grazioli P, Talora C, Frati L, Gulino A, Screpanti I. Bellavia D, et al. EMBO J. 2007 Mar 21;26(6):1670-80. doi: 10.1038/sj.emboj.7601626. Epub 2007 Mar 1. EMBO J. 2007. PMID: 17332745 Free PMC article. - Inhibition of p300/CBP by early B-cell factor.
Zhao F, McCarrick-Walmsley R, Akerblad P, Sigvardsson M, Kadesch T. Zhao F, et al. Mol Cell Biol. 2003 Jun;23(11):3837-46. doi: 10.1128/MCB.23.11.3837-3846.2003. Mol Cell Biol. 2003. PMID: 12748286 Free PMC article. - Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors.
Wu L, Sun T, Kobayashi K, Gao P, Griffin JD. Wu L, et al. Mol Cell Biol. 2002 Nov;22(21):7688-700. doi: 10.1128/MCB.22.21.7688-7700.2002. Mol Cell Biol. 2002. PMID: 12370315 Free PMC article. - NOTCH1-RBPJ complexes drive target gene expression through dynamic interactions with superenhancers.
Wang H, Zang C, Taing L, Arnett KL, Wong YJ, Pear WS, Blacklow SC, Liu XS, Aster JC. Wang H, et al. Proc Natl Acad Sci U S A. 2014 Jan 14;111(2):705-10. doi: 10.1073/pnas.1315023111. Epub 2013 Dec 27. Proc Natl Acad Sci U S A. 2014. PMID: 24374627 Free PMC article.
References
- Ait-Si-Ali S, Ramirez S, Barre FX, Dkhissi F, Magnaghi-Jaulin L, Girault JA, Robin P, Knibiehler M, Pritchard LL, Ducommun B, et al. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature. 1998;396:184–186. - PubMed
- Anderson A, Robey E, Huang Y. Notch signaling in lymphocyte development. Curr Op Genet Dev. 2001;11:554–560. - PubMed
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Bulger M, Kadonaga JT. Biochemical reconstitution of chromatin with physiological nucleosome spacing. Methods Mol Genet. 1994;5:242–262.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous