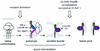Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates - PubMed (original) (raw)
Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates
Hana Golding et al. J Virol. 2002 Jul.
Abstract
Human immunodeficiency virus type 1 (HIV-1) entry requires conformational changes in the transmembrane subunit (gp41) of the envelope glycoprotein (Env) involving transient fusion intermediates that contain exposed coiled-coil (prehairpin) and six-helix bundle structures. We investigated the HIV-1 entry mechanism and the potential of antibodies targeting fusion intermediates to block Env-mediated membrane fusion. Suboptimal temperature (31.5 degrees C) was used to prolong fusion intermediates as monitored by confocal microscopy. After transfer to 37 degrees C, these fusion intermediates progressed to syncytium formation with enhanced kinetics compared with effector-target (E/T) cell mixtures that were incubated only at 37 degrees C. gp41 peptides DP-178, DP-107, and IQN17 blocked fusion more efficiently (5- to 10-fold-lower 50% inhibitory dose values) when added to E/T cells at the suboptimal temperature prior to transfer to 37 degrees C. Rabbit antibodies against peptides modeling the N-heptad repeat or the six-helix bundle of gp41 blocked fusion and viral infection at 37 degrees C only if preincubated with E/T cells at the suboptimal temperature. Similar fusion inhibition was observed with human six-helix bundle-specific monoclonal antibodies. Our data demonstrate that antibodies targeting gp41 fusion intermediates are able to bind to gp41 and arrest fusion. They also indicate that six-helix bundles can form prior to fusion and that the lag time before fusion occurs may include the time needed to accumulate preformed six-helix bundles at the fusion site.
Figures
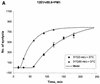
FIG. 1.
Enhanced kinetics of syncytium formation at 37°C after preincubation of E/T cells at 31.5°C. Effectors were 12E1 cells infected for 18 h with recombinant vaccinia virus expressing 89.6 (dual tropic) Env (A) or JR-FL (R5) Env (B). Effector and target (PM1) cells were mixed at a 1:1 ratio and either incubated at 37°C or preincubated at 31.5°C (A and B) or at 23°C (B, broken line) for 60 min before transfer to 37°C. (C) TF228 (IIIB Env) effector cells were mixed with target (PM1) cells and preincubated at 31.5°C for the indicated times before transfer to 37°C. No syncytia were scored at the time of transfer. The data were fitted according to the mathematical model described in Materials and Methods. Statistically significant differences in lag time and fusion rate constant were seen after 30 and 60 min of preincubation at 31.5°C (see Table 1).
FIG. 1.
Enhanced kinetics of syncytium formation at 37°C after preincubation of E/T cells at 31.5°C. Effectors were 12E1 cells infected for 18 h with recombinant vaccinia virus expressing 89.6 (dual tropic) Env (A) or JR-FL (R5) Env (B). Effector and target (PM1) cells were mixed at a 1:1 ratio and either incubated at 37°C or preincubated at 31.5°C (A and B) or at 23°C (B, broken line) for 60 min before transfer to 37°C. (C) TF228 (IIIB Env) effector cells were mixed with target (PM1) cells and preincubated at 31.5°C for the indicated times before transfer to 37°C. No syncytia were scored at the time of transfer. The data were fitted according to the mathematical model described in Materials and Methods. Statistically significant differences in lag time and fusion rate constant were seen after 30 and 60 min of preincubation at 31.5°C (see Table 1).
FIG. 1.
Enhanced kinetics of syncytium formation at 37°C after preincubation of E/T cells at 31.5°C. Effectors were 12E1 cells infected for 18 h with recombinant vaccinia virus expressing 89.6 (dual tropic) Env (A) or JR-FL (R5) Env (B). Effector and target (PM1) cells were mixed at a 1:1 ratio and either incubated at 37°C or preincubated at 31.5°C (A and B) or at 23°C (B, broken line) for 60 min before transfer to 37°C. (C) TF228 (IIIB Env) effector cells were mixed with target (PM1) cells and preincubated at 31.5°C for the indicated times before transfer to 37°C. No syncytia were scored at the time of transfer. The data were fitted according to the mathematical model described in Materials and Methods. Statistically significant differences in lag time and fusion rate constant were seen after 30 and 60 min of preincubation at 31.5°C (see Table 1).
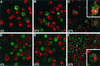
FIG. 2.
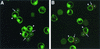
Confocal microscopy of effector/target cell conjugates. In panels A to C, TF228 (IIIB Env) and CEM cells were labeled with the membrane dyes PKH26 (red) and PKH67 (green), respectively. In panels D to F, TF228 cells were labeled with the cytoplasmic dye calcein AM (diffused green) and CEM cells were labeled with the membrane dye PKH26 (red). Cell mixtures were incubated for 2 h at 4°C (A and D), 31.5°C (B and E), or 37°C (C and F). Conjugates were analyzed by confocal microscopy. Three-dimensional (A and B) or two-dimensional (C to F) images are presented at either ×100 (A, B, D, and E) or ×40 (C and F) magnification. Insets in panels C and F show ×100 images of syncytia formed at 37°C, as indicated by yellow patches (C) or by arrowheads (F). Data represent several experiments.
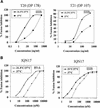
FIG. 3.
Increased potency of fusion inhibition mediated by gp41 peptides when added to E/T cells at 31.5°C. Serial dilutions of T20 and T21 (A) or IQN17 (B) were added to E/T cells that were either incubated at 37°C (▪) or preincubated with the inhibitors for 60 min at 31.5°C and then transferred to 37°C (♦). Syncytia were scored 3 to 4 h after transfer to 37°C. The data are plotted as percent inhibition of syncytia in control cultures (medium control) as a function of the inhibitor dose. Three wells per group were set up in all experiments, and the standard deviations did not exceed 15% of the means. The ID50 values calculated from the curves were as follows: T20, 37°C (65 ng/ml), 31.5°C (5 ng/ml); T21, 37°C (540 ng/ml), 31.5°C (65 ng/ml); IQN17 (89.6 Env), 37°C (400 nM), 31.5°C (50 nM); and IQN17 (IIIB Env), 37°C (120 nM), 31.5°C (28 nM).
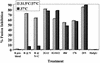
FIG. 4.
Inhibition of fusion with rabbit anti-N-HR and anti-N+C-HR-peptide antibodies when added to E/T cells at 31.5°C before transfer to 37°C. TF228 (IIIB Env)/PM1 cell mixtures were incubated with various human monoclonal antibodies (or human IgG [HuIgG] control) or with IgG from rabbits immunized with N-HR peptide (R288) or with N+C-HR peptide mixture (antibundle, R948) (or with preimmune rabbit IgG) at 10 μg/ml. The antibodies were preincubated with the E/T cell mixtures at 31.5°C for 1 h before transfer to 37°C (light columns) or only at 37°C (dark columns). In the case of human monoclonal antibodies, the monoclonal antibodies were incubated with the Env-expressing cells for 1 h at 37°C before addition of the target cells (dark columns).
FIG. 5.
Binding of the antibundle antibodies to fusion intermediates at E/T cell contacts. TF228 (IIIB Env) cells were labeled with calcein AM and cocultured with CEM cells labeled with PKH67 for 2 h at 31.5°C. IgG from a nonimmunized rabbit (A) or from the rabbit immunized with gp41-derived peptides (N36+C34) (B) was added to the cell cultures for the last hour of incubation. Cells were harvested, incubated with Alexa Fluor 546 goat anti-rabbit IgG, and mounted on slides. Digital images of the cell cultures are presented as two-dimensional images scanned at ×100 magnification. CEM cells are marked with arrowheads, TF228 cells are marked with asterisks, and E/T cell junctions are indicated with arrows. The presence of red color in the E/T cell junction areas indicates binding of antibundle antibodies.
FIG. 6.
Preincubation of E/T cells at 31.5°C before transfer to 37°C results in reduced susceptibility to inhibition by T20 peptide. Serial dilutions of T20 were added to E/T cells that were incubated at 37°C (▪) or preincubated with the inhibitor for 60 min at 31.5°C and then transferred to 37°C (⧫) or preincubated without the inhibitor at 31.5°C for 60 min. T20 was added to these cells at the time of transfer to 37°C (▴). Syncytia were scored 3 h (89.6 Env) or 4 h (IIIB Env) after transfer to 37°C. The data are plotted as percent inhibition of syncytia in control cultures (medium control) as a function of the inhibitor dose. Three wells per group were set up in all experiments, and the standard deviations did not exceed 15% of the means. The ID50 values calculated from the curves were as follows: 89.6 Env, 11 ng/ml (▪), 1.5 ng/ml (⧫), and 250 ng/ml (▴); IIIB Env, 5.2 ng/ml (▪), 0.4 ng/ml (⧫), and 190 ng/ml (▴).
FIG. 7.
Dynamics of the transition from six-helix bundles to membrane fusion: loss of sensitivity to neutralization by antibundle antibodies after transfer of E/T cells from 31.5°C (2 h) to 37°C in the presence of anti-CD4 monoclonal antibody. TF228 (IIIB Env)/PM1 cells were incubated for 2 h at 31.5°C in the absence of antibodies (Abs). Immediately prior to transfer to 37°C (time zero), anti-CD4 monoclonal antibody Q4120 was added at 3 μg/ml. Six-helix bundle-specific IgG from R948 was added at 30 μg/ml at the indicated times. Syncytia were scored 4 h after transfer to 37°C.
FIG. 8.
Proposed model of gp41 conformational changes leading to fusion. Arrows indicate potential binding of peptides and antibodies (Ab) targeting the prehairpin and six-helix bundle fusion intermediates.
Similar articles
- Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion.
Finnegan CM, Berg W, Lewis GK, DeVico AL. Finnegan CM, et al. J Virol. 2002 Dec;76(23):12123-34. doi: 10.1128/jvi.76.23.12123-12134.2002. J Virol. 2002. PMID: 12414953 Free PMC article. - Membrane-anchored inhibitory peptides capture human immunodeficiency virus type 1 gp41 conformations that engage the target membrane prior to fusion.
Melikyan GB, Egelhofer M, von Laer D. Melikyan GB, et al. J Virol. 2006 Apr;80(7):3249-58. doi: 10.1128/JVI.80.7.3249-3258.2006. J Virol. 2006. PMID: 16537592 Free PMC article. - Early steps of HIV-1 fusion define the sensitivity to inhibitory peptides that block 6-helix bundle formation.
Miyauchi K, Kozlov MM, Melikyan GB. Miyauchi K, et al. PLoS Pathog. 2009 Sep;5(9):e1000585. doi: 10.1371/journal.ppat.1000585. Epub 2009 Sep 18. PLoS Pathog. 2009. PMID: 19763181 Free PMC article. - HIV-1 gp41: mediator of fusion and target for inhibition.
Weiss CD. Weiss CD. AIDS Rev. 2003 Oct-Dec;5(4):214-21. AIDS Rev. 2003. PMID: 15012000 Review. - Biochemistry and biophysics of HIV-1 gp41 - membrane interactions and implications for HIV-1 envelope protein mediated viral-cell fusion and fusion inhibitor design.
Cai L, Gochin M, Liu K. Cai L, et al. Curr Top Med Chem. 2011 Dec;11(24):2959-84. doi: 10.2174/156802611798808497. Curr Top Med Chem. 2011. PMID: 22044229 Free PMC article. Review.
Cited by
- Design of a modular tetrameric scaffold for the synthesis of membrane-localized D-peptide inhibitors of HIV-1 entry.
Francis JN, Redman JS, Eckert DM, Kay MS. Francis JN, et al. Bioconjug Chem. 2012 Jun 20;23(6):1252-8. doi: 10.1021/bc300076f. Epub 2012 May 17. Bioconjug Chem. 2012. PMID: 22545664 Free PMC article. - The cytoplasmic tail slows the folding of human immunodeficiency virus type 1 Env from a late prebundle configuration into the six-helix bundle.
Abrahamyan LG, Mkrtchyan SR, Binley J, Lu M, Melikyan GB, Cohen FS. Abrahamyan LG, et al. J Virol. 2005 Jan;79(1):106-15. doi: 10.1128/JVI.79.1.106-115.2005. J Virol. 2005. PMID: 15596806 Free PMC article. - Time frames for neutralization during the human immunodeficiency virus type 1 entry phase, as monitored in synchronously infected cell cultures.
Haim H, Steiner I, Panet A. Haim H, et al. J Virol. 2007 Apr;81(7):3525-34. doi: 10.1128/JVI.02293-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251303 Free PMC article. - Recruitment of HIV-1 envelope occurs subsequent to lipid mixing: a fluorescence microscopic evidence.
Chien MP, Lin CH, Chang DK. Chien MP, et al. Retrovirology. 2009 Mar 2;6:20. doi: 10.1186/1742-4690-6-20. Retrovirology. 2009. PMID: 19254359 Free PMC article. - The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design.
Montero M, van Houten NE, Wang X, Scott JK. Montero M, et al. Microbiol Mol Biol Rev. 2008 Mar;72(1):54-84, table of contents. doi: 10.1128/MMBR.00020-07. Microbiol Mol Biol Rev. 2008. PMID: 18322034 Free PMC article. Review.
References
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
- Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. - PubMed
- Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. - PubMed
- Chen, C. H., M. L. Greenberg, D. P. Bolognesi, and T. J. Matthews. 1995. Monoclonal antibodies that bind to the core of fusion-active glycoprotein 41. J. Virol. 69:1462-1472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources