Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation - PubMed (original) (raw)
Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation
Young Kyeung Kim et al. Mol Cell Biol. 2002 Jul.
Abstract
Stimulation of transcriptional elongation by the human immunodeficiency virus type 1 Tat protein is mediated by CDK9, a kinase that phosphorylates the RNA polymerase II carboxyl-terminal domain (CTD). In order to obtain direct evidence that this phosphorylation event can alter RNA polymerase processivity, we prepared transcription elongation complexes that were arrested by the lac repressor. The CTD was then dephosphorylated by treatment with protein phosphatase 1. The dephosphorylated transcription complexes were able to resume the transcription elongation when IPTG (isopropyl-beta-D-thiogalactopyranoside) and nucleotides were added to the reaction. Under these chase conditions, efficient rephosphorylation of the CTD was observed in complexes containing the Tat protein but not in transcription complexes prepared in the absence of Tat protein. Immunoblots and kinase assays with synthetic peptides showed that Tat activated CDK9 directly since the enzyme and its cyclin partner, cyclin T1, were present at equivalent levels in transcription complexes prepared in the presence or absence of Tat. Chase experiments with the dephosphorylated elongation transcription complexes were performed in the presence of the CDK9 kinase inhibitor DRB (5,6-dichloro-1-beta-D-ribofuranosyl-benzimidazole). Under these conditions there was no rephosphorylation of the CTD during elongation, and transcription through either a stem-loop terminator or bent DNA arrest sequence was strongly inhibited. In experiments in which the CTD was phosphorylated prior to elongation, the amount of readthrough of the terminator sequences was proportional to the extent of the CTD modification. The change in processivity is due to CTD phosphorylation alone, since even after the removal of Spt5, the second substrate for CDK9, RNA polymerase elongation is enhanced by Tat-activated CDK9 activity. We conclude that phosphorylation of the RNA polymerase II CTD by CDK9 enhances transcription elongation directly.
Figures
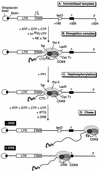
FIG. 1.
Strategy used for analyzing transcription elongation complexes. (A) Structure of HIV-LTR template. DNA templates containing the lac operator (lacO) binding site for the lac repressor protein (LacR) and a terminator (τ) sequence were biotinylated and bound to streptavidin beads. (B) Elongation complexes were trapped by the lac repressor (LacR) after incubation of the immobilized templates with HeLa nuclear extract (NE) in the presence of nucleotide triphosphates and LacR and in the absence or presence of Tat. The CTD of the RNA polymerase was phosphorylated during the elongation reaction due to the activity of CDK7 and CDK9. (C) Elongation complexes arrested by LacR were treated with PP1 to remove phosphate groups from the CTD. (D) The phosphatase-treated complexes can resume transcription elongation after the addition of nucleotides and IPTG. During the chase reaction the CTD became phosphorylated by CDK9. The addition of DRB blocked the rephosphorylation of the CTD and induced pausing of the transcription complex at the terminator sequences.
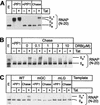
FIG. 2.
Tat and TAR stimulate hyperphosphorylation of CTD in transcription elongation complexes. (A) Rephosphorylation by CDK9. Elongation complexes assembled on the pW1 templates were dephosphorylated by PP1 treatment (Pol IIa). Chase of the dephosphorylated complexes from the LacR site in the presence of all four nucleotides and IPTG permits rephosphorylation of the RNA polymerase CTD (Pol IIo*) in the presence (+) of 20 ng of Tat but not in the absence (−) of Tat. (B) Inhibition of CDK9 by DRB. Between 0 and 10 μM DRB was included in the chase reactions containing dephosphorylated elongation complexes. Transcription was performed on the pW1 DNA templates in the absence (−) and presence (+) of 20 ng of Tat. (C) Activation of CDK9 by Tat and TAR. Elongation complexes were assembled on templates carrying wild-type TAR (WT) or mutant TAR elements in the Tat-binding site (mGC) or in the CycT1-binding site (mLG) in the absence (−) or presence (+) of 20 ng of Tat. After dephosphorylation of RNA polymerase CTD, the complexes were chased as described above.
FIG. 3.
CDK9 phosphorylates Ser5 and Ser2 of the CTD in elongation complexes. (A) Transcription reactions. Preinitiation complexes (PIC) were assembled on immobilized wild-type template (WT) by using hexokinase/glucose-treated HeLa nuclear extract in the presence of 50 μM dATP and in the absence (−) or presence (+) of 20 ng of Tat. Transcription complexes paused at the uridine residue at position 14 were obtained after elongation of the preinitiation complexes in the absence of ATP. Standard transcription reactions were performed in parallel with templates carrying either the wild-type TAR element (WT) or a mutation in the Tat-binding site (mGC). Protein composition and phosphorylation of RNA Pol II CTD were analyzed from different transcription complexes by immunoblotting with the N-20, H5, and H14 antibodies directed against RNA Pol II (RNAP) and antibodies against CDK9, CycT1, CDK7, and CycH. (B) Rephosphorylation reactions. Transcription elongation complexes arrested by LacR were dephosphorylated by PP1, washed with EBCD buffer containing 0.1% Sarkosyl, and chased in the presence of 25 mM IPTG and all four nucleotide triphosphates. The proteins were detected by immunoblotting with the N-20, 8WG16, H5, and H14 antibodies directed against RNA Pol II and antibodies against CDK9 and CycT1.
FIG. 4.
Activation of P-TEFb by Tat. In vitro kinase reactions were performed by using P-TEFb purified by immunoprecipitation from HeLa nuclear extract or CDK9 in transcription elongation complexes arrested by LacR. The kinase reactions were performed using a peptide carrying 5 heptad repeat sequences (CTD5) as the substrate. Reactions were performed in the presence of 1 μCi of [γ-32P]ATP and in the absence (−) or presence (+) of 2 μM cold ATP, 2.5 mM MnCl2, and 20 ng of Tat. The phosphorylated peptides were separated by SDS-4 to 20% PAGE, transferred onto nitrocellulose membranes, and detected by autoradiography.
FIG. 5.
Protease mapping of CTD phosphorylation sites in elongation complexes. (A) CTD domain. The diagram shows the position of protease cleavage sites for Asp-N, Glu-C, and Lys-C. Asp-N cleaves peptide bonds N terminally at aspartic acids (D). Glu-C cleaves peptide bonds at the carboxyl side of glutamic acids (E), as well as at the carboxyl side of aspartic acids (D). Cleavage at E* by Glu-C was prevented by the proline residue on the carboxyl side. Lys-C specifically cleaves peptide bonds on the carboxyl-terminal side of lysine (K) residues. Cleavage products of each protease are shown as shaded bars. (B) Elongation complexes. LacR-arrested and dephosphorylated elongation complexes were labeled with 10 μCi of [γ-32P]ATP as described in the text. The 32P-labeled RNA Pol II was immunoprecipitated with N-20 antibody and digested by the indicated proteases. The cleavage products were separated on SDS-4 to 12% PAGE gels, transferred to nitrocellulose membranes, and detected by autoradiography. (C) Glu-C digest. Preinitiation and/or elongation complexes were prepared with CDK7-, CDK9-, or mock-depleted HeLa nuclear extracts. RNA Pol II from the complexes was labeled with 10 μCi of [γ-32P]ATP, purified by immunoprecipitation, and digested with Glu-C. The labeled peptides were separated in SDS-4 to 12% PAGE gels, transferred to nitrocellulose membranes, and then detected by autoradiography. (D) Immunoblot analysis of CDK9- or mock-depleted extracts (left panel) and CDK7- or mock-depleted extracts (right panel). Note that CDK9 depletion did not affect the levels of CDK7 or RNA Pol II and that CDK7 depletion did not change the levels of CDK8, CDK9, or RNA Pol II.
FIG. 6.
Phosphorylation of the CTD is required for efficient transcription through terminator sequences. (A) Structure of templates. The pW1 template carries a terminator formed by an RNA stem-loop, followed by nine uridines (τ). pH3.3 contains two tandem arrest sequences (τ′1a and τ′1b) that induce DNA bending. In pΔTerm, the terminator sequence was replaced by the original HIV sequence. (B) Transcription reactions were performed with three different immobilized DNA templates (pW1, pH3.3, or pΔTerm). Reactions contained 100 ng of LacR and were performed in the absence (−) or presence (+) of 20 ng of Tat. After labeling for 20 min with [α-32P]UTP (−PP1), the immobilized templates were purified and treated with RNase H in the presence of the RHX1 and RHLAC oligonucleotides to remove the labeled RNA transcripts from transcription complexes that had read through the LacR site. The arrested complexes were then dephosphorylated by PP1 treatment (+PP1). After the complexes were washed with TMZ buffer, the dephosphorylated complexes were chased by the addition of 250 μM ATP, GTP, and CTP; 5 μM UTP; and 25 mM IPTG in the absence (−) or presence (+) of 100 μM DRB. Positions of transcripts at the runoffs (ρ), terminator (τ), and lac repressor (LacR) in pW1 template are indicated. τ′1a and τ′1b indicate the stop sites from the pH3.3 template. (C) Immunoblot. Samples of the reactions shown in panel B were immunoblotted with the N-20 antibody against RNA Pol II and the anti-Spt5 antibody. Tat-dependent hyperphosphorylation of RNA polymerase CTD and Spt5 was observed during the chase of dephosphorylated complexes assembled on each template.
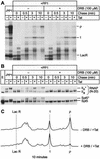
FIG. 7.
CTD phosphorylation by CDK9 enhances processivity of RNA Pol II. (A) Elongation complexes were assembled in the presence of LacR with HeLa nuclear extract on the pW1 DNA template and treated with RNase H and protein phosphatase. The complexes were then chased for the indicated times (0, 0.5, 3, and 10 min) in the absence (−) or presence (+) of DRB. RNA polymerase processivity is increased by the phosphorylation of CTD in the presence of Tat, but the addition of the CDK9 inhibitor DRB abolishes the activation of RNA Pol II processivity by Tat. (B) Immunoblots showing phosphorylation extent of RNA Pol II and Spt5 were performed with N-20 antibody against RNA Pol II or anti-Spt5 antibody. (C) Quantitative analysis of the gel shown in panel A by densitometry. The changes in the distributions of transcripts produced by the rephosphorylated transcription complexes (−DRB/+Tat) and unphosphorylated transcription complexes (+DRB/+Tat) during the chase reactions provide a direct measure of changes in RNA polymerase processivity.
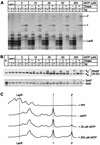
FIG. 8.
Activation of elongation complexes by phosphorylation prior to elongation. (A) Transcription. Elongation complexes were assembled by using the pW1 DNA template in the presence of lac repressor and dephosphorylated (+PP1) as described in the legend to Fig. 6. The complexes were rephosphorylated by adding between 0 and 250 μM dATP as a phosphate donor. After a brief wash to remove unincorporated dATP, 100 μM DRB was then added to prevent further phosphorylation of the complex during the chase reaction. The rephosphorylated complexes were chased by addition of all four ribonucleotide triphosphates and 25 mM IPTG. Positions of transcripts at the runoff (ρ), terminator (τ), and lac repressor (LacR) are shown. (B) Immunoblot. Samples from the reactions shown in panel A were immunoblotted with N-20 antibody against RNA Pol II and the anti-Spt5 antibody. (C) Quantitative analysis of the gel shown in panel A by densitometry. Note that there is a strong correlation between the extent of CTD phosphorylation and the amount of the runoff product (ρ).
FIG. 9.
Spt5 is not required for Tat-dependent activation of elongation and RNA polymerase phosphorylation. (A) Transcription. Elongation complexes were assembled in the presence of lac repressor (−PP1), dephosphorylated (+PP1), and then chased with either mock-depleted or Spt5-depleted HeLa nuclear extracts. Reactions were performed with either the pW1 template or the pH3.3 template as described in the legend to Fig. 6. Positions of transcripts at the runoff (ρ), terminator (τ, τ′1a, and τ′1b), and lac repressor (LacR) are shown. (B) Immunoblot. Samples from the reactions shown in panel A were immunoblotted with N-20 antibody against RNA Pol II and the anti-Spt5 antibody. Note that hyperphosphorylated polymerases can elongate efficiently through both the terminator site and the arrest sites even in the absence of Spt5.
Similar articles
- Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription.
Zhou M, Halanski MA, Radonovich MF, Kashanchi F, Peng J, Price DH, Brady JN. Zhou M, et al. Mol Cell Biol. 2000 Jul;20(14):5077-86. doi: 10.1128/MCB.20.14.5077-5086.2000. Mol Cell Biol. 2000. PMID: 10866664 Free PMC article. - Spt5 cooperates with human immunodeficiency virus type 1 Tat by preventing premature RNA release at terminator sequences.
Bourgeois CF, Kim YK, Churcher MJ, West MJ, Karn J. Bourgeois CF, et al. Mol Cell Biol. 2002 Feb;22(4):1079-93. doi: 10.1128/MCB.22.4.1079-1093.2002. Mol Cell Biol. 2002. PMID: 11809800 Free PMC article. - Regulatory functions of Cdk9 and of cyclin T1 in HIV tat transactivation pathway gene expression.
Romano G, Kasten M, De Falco G, Micheli P, Khalili K, Giordano A. Romano G, et al. J Cell Biochem. 1999 Dec 1;75(3):357-68. J Cell Biochem. 1999. PMID: 10536359 Review. - CDK9: from basal transcription to cancer and AIDS.
De Falco G, Giordano A. De Falco G, et al. Cancer Biol Ther. 2002 Jul-Aug;1(4):342-7. Cancer Biol Ther. 2002. PMID: 12432243 Review.
Cited by
- Cocaine-induced DNA-PK relieves RNAP II pausing by promoting TRIM28 phosphorylation.
Sharma AL, Tyagi P, Khumallambam M, Tyagi M. Sharma AL, et al. bioRxiv [Preprint]. 2024 Aug 19:2024.08.19.608673. doi: 10.1101/2024.08.19.608673. bioRxiv. 2024. PMID: 39229050 Free PMC article. Preprint. - KAP1 negatively regulates RNA polymerase II elongation kinetics to activate signal-induced transcription.
Hyder U, Challa A, Thornton M, Nandu T, Kraus WL, D'Orso I. Hyder U, et al. Nat Commun. 2024 Jul 12;15(1):5859. doi: 10.1038/s41467-024-49905-7. Nat Commun. 2024. PMID: 38997286 Free PMC article. - KAP1 negatively regulates RNA polymerase II elongation kinetics to activate signal-induced transcription.
Hyder U, Challa A, Thornton M, Nandu T, Kraus WL, D'Orso I. Hyder U, et al. bioRxiv [Preprint]. 2024 May 5:2024.05.05.592422. doi: 10.1101/2024.05.05.592422. bioRxiv. 2024. PMID: 38746145 Free PMC article. Updated. Preprint. - Bioinformatics Insights on Viral Gene Expression Transactivation: From HIV-1 to SARS-CoV-2.
Patarca R, Haseltine WA. Patarca R, et al. Int J Mol Sci. 2024 Mar 16;25(6):3378. doi: 10.3390/ijms25063378. Int J Mol Sci. 2024. PMID: 38542351 Free PMC article. - CDK9-55 guides the anaphase-promoting complex/cyclosome (APC/C) in choosing the DNA repair pathway choice.
Alfano L, Iannuzzi CA, Barone D, Forte IM, Ragosta MC, Cuomo M, Mazzarotti G, Dell'Aquila M, Altieri A, Caporaso A, Roma C, Marra L, Boffo S, Indovina P, De Laurentiis M, Giordano A. Alfano L, et al. Oncogene. 2024 Apr;43(17):1263-1273. doi: 10.1038/s41388-024-02982-w. Epub 2024 Mar 4. Oncogene. 2024. PMID: 38433256
References
- Akoulitchev, S., S. Chuikov, and D. Reinberg. 2000. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407:102-106. - PubMed
- Akoulitchev, S., T. P. Mäkelä, R. A. Weinberg, and D. Reinberg. 1995. Requirement for TFIIH kinase activity in transcription by RNA polymerase II. Nature 377:557-560. - PubMed
- Awrey, D. E., R. G. Weilbaecher, S. A. Hemming, S. M. Orlicky, C. M. Kane, and A. M. Edwards. 1997. Transcription elongation through DNA arrest sites: a multistep process involving both RNA polymerase II subunit RPB9 and TFIIS. J. Biol. Chem. 272:14747-14754. - PubMed
- Bienkiewicz, E. A., A.-Y. M. Woody, and R. W. Woody. 2000. Conformation of the RNA polymerase II C-terminal domain: circular dichroism of long and short fragments. J. Mol. Biol. 297:119-133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous