Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability - PubMed (original) (raw)
Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability
Shannon A Kuismanen et al. Am J Pathol. 2002 Jun.
Abstract
The colorectum and uterine endometrium are the two most commonly affected organs in hereditary nonpolyposis colon cancer (HNPCC), but the genetic basis of organ selection is poorly understood. As tumorigenesis in HNPCC is driven by deficient DNA mismatch repair (MMR), we compared its typical consequence, instability at microsatellite sequences, in colorectal and endometrial cancers from patients with identical predisposing mutations in the MMR genes MLH1 or MSH2. Analysis of non-coding (BAT25, BAT26, and BAT40) and coding mononucleotide repeats (MSH6, MSH3, MLH3, BAX, IGF2R, TGF beta RII, and PTEN), as well as MLH1- and MSH2-linked dinucleotide repeats (D3S1611 and CA7) revealed significant differences, both quantitative and qualitative, between the two tumor types. Whereas colorectal cancers displayed a predominant pattern consisting of instability at the BAT loci (in 89% of tumors), TGF beta RII (73%), dinucleotide repeats (70%), MSH3 (43%), and BAX (30%), no such single pattern was discernible in endometrial cancers. Instead, the pattern was more heterogeneous and involved a lower proportion of unstable markers per tumor (mean 0.27 for endometrial cancers versus 0.45 for colorectal cancers, P < 0.001) and shorter allelic shifts for BAT markers (average 5.1 bp for unstable endometrial cancers versus 9.3 bp for colorectal cancers, P < 0.001). Among the individual putative "target" loci, PTEN instability was associated with endometrial cancers and TGF beta RII instability with colon cancers. The different instability profiles in endometrial and colorectal cancers despite identical genetic predisposition underlines organ-specific differences that may be important determinants of the HNPCC tumor spectrum.
Figures
Figure 1.
Comparison of the MSI profiles in colorectal (A) and endometrial (B) cancers originating from MLH1 or MSH2 mutation carriers. Mutations 1–8 affect MLH1 while mutation 9 affects MSH2. The expression patterns of the proteins corresponding to the genes mutated in the germline (MLH1 for cases with mutations 1–8, MSH2 for cases with mutation 9), as determined by immunohistochemistry (IHC), are shown on the right. Case F67:6 in A is a colorectal adenoma. Eight patients were diagnosed with both endometrial and colorectal cancer, and the identification numbers of these individuals are in italics. The tumors were grouped into four categories according to their BAT and coding region instability patterns (among colorectal cancers, there were no cases in the “coding region instability only” category). Among genes with coding repeats, MSH6, MSH3, and MLH3 are MMR genes, BAX is a proapoptotic gene, and IGF2R, TGFβRII, and PTEN are tumor suppressor genes. The percentage of (informative) tumors showing instability for each marker is indicated below the marker columns. MSI: black, unstable; white, stable; gray, not determined. IHC: diagonal stripes, not expressed; gray, not determined.
Figure 2.
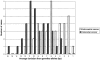
Distribution of allelic size shifts in colorectal versus endometrial tumors. The values on the x axis represent the means of size deviations at the BAT25, BAT26, and BAT40 loci from the length of the germline alleles, calculated for each tumor and plotted against the number of cases. All size shifts were deletions.
Figure 3.
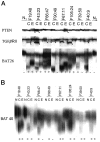
A: Autoradiographs of mononucleotide repeat instability analysis of the PTEN (exon 7), TGFβRII, and BAT26 loci in paired cases of colorectal (C) and endometrial (E) tumors from the same patients. These loci are essentially monomorphic, and the position of the normal-sized fragment can be determined from lanes N1 and N2 that represent normal DNA samples from control individuals. Arrowheads denote fragments of aberrant size resulting from gains (endometrial tumor from F19:48, TGFβRII) or losses of mononucleotides (all other unstable cases, PTEN and TGFβRII). Shortening of the A26 repeat within BAT26 gives rise to a ladder of extra fragments in the lower portion of the autoradiograph (+) that are absent in stable cases (−). B: Autoradiograph of BAT40 results, coded (+) or (−) as BAT26 above. Since BAT40 is polymorphic, the results from normal DNA of each individual (N) are shown adjacent to tumor lanes.
Similar articles
- Extended microsatellite analysis in microsatellite stable, MSH2 and MLH1 mutation-negative HNPCC patients: genetic reclassification and correlation with clinical features.
Schiemann U, Müller-Koch Y, Gross M, Daum J, Lohse P, Baretton G, Muders M, Mussack T, Kopp R, Holinski-Feder E. Schiemann U, et al. Digestion. 2004;69(3):166-76. doi: 10.1159/000078223. Epub 2004 Apr 28. Digestion. 2004. PMID: 15118395 - [The first molecular analysis of a Hungarian HNPCC family: a novel MSH2 germline mutation].
Czakó L, Tiszlavicz L, Takács R, Baradnay G, Lonovics J, Cserni G, Závodná K, Bartosova Z. Czakó L, et al. Orv Hetil. 2005 May 15;146(20):1009-16. Orv Hetil. 2005. PMID: 15945244 Hungarian. - Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German Hereditary Nonpolyposis Colorectal Cancer Consortium.
Plaschke J, Engel C, Krüger S, Holinski-Feder E, Pagenstecher C, Mangold E, Moeslein G, Schulmann K, Gebert J, von Knebel Doeberitz M, Rüschoff J, Loeffler M, Schackert HK. Plaschke J, et al. J Clin Oncol. 2004 Nov 15;22(22):4486-94. doi: 10.1200/JCO.2004.02.033. Epub 2004 Oct 13. J Clin Oncol. 2004. PMID: 15483016 - Molecular genetics and endometrial cancer.
Lalloo F, Evans G. Lalloo F, et al. Best Pract Res Clin Obstet Gynaecol. 2001 Jun;15(3):355-63. doi: 10.1053/beog.2000.0181. Best Pract Res Clin Obstet Gynaecol. 2001. PMID: 11476558 Review. - Carcinogenesis in the GI tract: from morphology to genetics and back again.
Redston M. Redston M. Mod Pathol. 2001 Mar;14(3):236-45. doi: 10.1038/modpathol.3880292. Mod Pathol. 2001. PMID: 11266532 Review.
Cited by
- BaxΔ2 is a novel bax isoform unique to microsatellite unstable tumors.
Haferkamp B, Zhang H, Lin Y, Yeap X, Bunce A, Sharpe J, Xiang J. Haferkamp B, et al. J Biol Chem. 2012 Oct 5;287(41):34722-9. doi: 10.1074/jbc.M112.374785. Epub 2012 Aug 21. J Biol Chem. 2012. PMID: 22910913 Free PMC article. - BaxΔ2 Family Alternative Splicing Salvages Bax Microsatellite-Frameshift Mutations.
Haferkamp B, Zhang H, Kissinger S, Wang X, Lin Y, Schultz M, Xiang J. Haferkamp B, et al. Genes Cancer. 2013 Nov;4(11-12):501-12. doi: 10.1177/1947601913515906. Genes Cancer. 2013. PMID: 24386510 Free PMC article. - Importance of PCR-based Tumor Testing in the Evaluation of Lynch Syndrome-associated Endometrial Cancer.
Bruegl AS, Kernberg A, Broaddus RR. Bruegl AS, et al. Adv Anat Pathol. 2017 Nov;24(6):372-378. doi: 10.1097/PAP.0000000000000169. Adv Anat Pathol. 2017. PMID: 28820751 Free PMC article. Review. - Canonical TGFβ Signaling and Its Contribution to Endometrial Cancer Development and Progression-Underestimated Target of Anticancer Strategies.
Zakrzewski PK. Zakrzewski PK. J Clin Med. 2021 Aug 30;10(17):3900. doi: 10.3390/jcm10173900. J Clin Med. 2021. PMID: 34501347 Free PMC article. Review.
References
- Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomäki P, Mecklin J-P, Järvinen H: Cancer risk in mutation carriers of DNA mismatch repair genes. Int J Cancer 1999, 81:214-218 - PubMed
- Parsons R, Li G-M, Longley M, Modrich P, Liu B, Berk T, Hamilton SR, Kinzler KW, Vogelstein B: Mismatch repair deficiency in phenotypically normal human cells. Science 1995, 268:738-740 - PubMed
- Vasen HFA, Wijnen JT, Menko FH, Kleibeuker JH, Taal BG, Griffioen G, Nagengast FM, Meijers-Heijboer EH, Bertario L, Varesco L, Bisgaard M-L, Mohr J, Fodde R, Meera Khan P: Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 1996, 110:1020-1027 - PubMed
- Wijnen J, de Leeuw W, Vasen H, van der Klift H, Møller P, Stormorken A, Meijers-Heijboer H, Lindhout D, Menko F, Vossen S, Möslein G, Tops C, Bröcker-Vriends A, Wu Y, Hofstra R, Sijmons R, Cornelisse C, Morreau H, Fodde R: Familial endometrial cancer in female carriers of MSH6 germline mutations. Nat Genet 1999, 23:142-144 - PubMed
- Prolla TA, Baker SM, Harris AC, Tsao JL, Yao X, Bronner CE, Zheng B, Gordon M, Reneker J, Arnheim N, Shibata D, Bradley A, Liskay RM: Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1, and Pms2 DNA mismatch repair. Nat Genet 1998, 18:276-279 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA067941/CA/NCI NIH HHS/United States
- U01 CA067941/CA/NCI NIH HHS/United States
- CA67941/CA/NCI NIH HHS/United States
- CA82282/CA/NCI NIH HHS/United States
- P30 CA16058/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous