Increased localization and substrate activation of protein kinase C delta in lung epithelial cells following exposure to asbestos - PubMed (original) (raw)
Increased localization and substrate activation of protein kinase C delta in lung epithelial cells following exposure to asbestos
Karen M Lounsbury et al. Am J Pathol. 2002 Jun.
Abstract
The protein kinase C (PKC) family consists of several isozymes whose substrates may be necessary for the regulation of key cellular events important in the pathogenesis of proliferative diseases. Asbestos is a carcinogen and fibroproliferative agent in lung that may cause cell signaling events through activation of PKC. Here we used a murine inhalation model of asbestos-induced inflammation and fibrosis to examine immunoreactivity of PKC delta and its substrate, phosphorylated-adducin (p-adducin), in cells of the lung. Moreover, we characterized PKC delta and p-adducin expression in a pulmonary epithelial cell line (C10) in both log versus confluent cells and in cells after mechanical wounding or crocidolite asbestos exposure. Both PKC delta and p-adducin were almost exclusively expressed in bronchiolar and alveolar type II (ATII) epithelial cells in lung sections and increased in these cell types after inhalation of asbestos by mice. Increases in membrane and nuclear localization of PKC delta were seen in log phase as compared to confluent C10 cells. Moreover, enhanced immunoreactivity of PKC delta was observed in epithelial cells expressing proliferating cell nuclear antigen (PCNA) after mechanical wounding or exposure to asbestos fibers. These studies show that activated PKC delta in pulmonary epithelial cells is a consequence of inhalation of asbestos and may be linked to the activation of cell proliferation.
Figures
Figure 1.
Increased expression of PKCδ and p-adducin in lungs treated with asbestos. PKCδ (A–C) and p-adducin (D–F) were detected in lung sections using immunoperoxidase staining of lung tissue sections. PKCδ: 30-day sham, A; 30-day asbestos exposure, B; 30-day asbestos exposure, C. p-adducin: 30-day sham, D; 30-day asbestos exposure, E; 30-day asbestos exposure, F. Note localization of PKCδ and p-adducin (arrows) in bronchiolar and alveolar epithelial cells and lesions (arrowheads) after 30 days of asbestos exposure; 30-day asbestos exposure (negative staining control using isotype control antibody), G; 30-day asbestos exposure (staining control omitting primary antibody), H. Original magnification, ×400 (A, B, D, E, G, H); magnification, ×200 (C and F).
Figure 2.
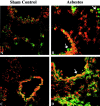
PKCδ and p-adducin co-localize within bronchiolar epithelial cells after asbestos exposure. Representative images illustrating co-localization of PKCδ (green) and p-adducin (red) using immunofluorescence in lung tissue sections. A: 4-day sham exposure. B: 4-day asbestos exposure. C: 4-day asbestos exposure (staining control omitting primary antibodies). D: 30-day sham exposure. E: 30-day asbestos exposure. F: 30-day asbestos exposure (staining control omitting primary antibodies). Original magnification, ×400.
Figure 3.
PKCδ is predominantly expressed in epithelial cells and not in macrophages. Representative images illustrating co-localization of PKCδ antibody (red) with MAC-3 (A, B) or CytoKeratin7 (C, D) antibodies (green) using immunofluorescence in lung tissue sections from sham control (A, C) or 30-day crocidolite asbestos exposed animals (B, D). Arrows in B show MAC-3 staining macrophages with no PKCδ reactivity Arrows in D show co-localization of PKCδ in bronchiolar epithelial cells stained with Cytokeratin7. Original magnification, ×400.
Figure 4.
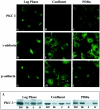
PKCδ and p-adducin are localized to the membrane and nuclei of dividing cells or confluent cells treated with PDBu. C10 cells were examined at low density (Log Phase), confluent density (Confluent) and after treatment with 100 nmol/L PDBu for 10 minutes (PDBu). A–I: Localization of proteins by immunofluorescence using antibodies to PKCδ (A–C); γ-adducin (D–F); and p-adducin (G–I). Original magnification, ×600. J: Localization of PKCδ in subcellular fractions of C10 cells by Western blot. Tot, total extract; M, membrane fraction; C, cytosolic fraction; N, nuclear fraction.
Figure 5.
PKCδ and p-adducin are localized to the membrane and nuclei of cells migrating into a wound. Confluent C10 monolayers were wounded by a rubber policeman then allowed to respond for 24 hours. Cells were then fixed and immunostained as in Figure 4 ▶ using antibodies recognizing PKCδ (A), p-adducin (B), and γ-adducin (C). D: Staining control omitting primary antibody. Original magnification, ×400.
Figure 6.
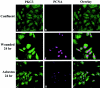
Cells exhibiting increased translocalization of PKCδ after wounding or asbestos exposure also exhibit an increase in PCNA. C10 cells were wounded as in Figure 5 ▶ (D–F) or exposed to asbestos (5 μg/cm2-area dish) for 24 hours (G–I). Cells were then co-stained by immunofluorescence using antibodies recognizing PKCδ (green) and PCNA (magenta). Asbestos fibers are shown in red. C and F represent overlay image of PKCδ and PCNA for wounded and asbestos exposed cells respectively. Original magnification, ×400.
Figure 7.
Increased localization of PKCδ and p-adducin in focal regions of asbestos fiber deposition. Control C10 cells (A) and cells exposed to 5 μg/cm2 area dish of asbestos for 24 hours (B, C) were examined by immunofluorescence (green) for the localization of PKCδ (A, B) and p-adducin (C). Asbestos fibers are shown in red. Original magnification, ×400.
Similar articles
- Asbestos-induced apoptosis is protein kinase C delta-dependent.
Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Shukla A, et al. Am J Respir Cell Mol Biol. 2003 Aug;29(2):198-205. doi: 10.1165/rcmb.2002-0248OC. Epub 2003 Mar 6. Am J Respir Cell Mol Biol. 2003. PMID: 12626342 - Persistent localization of activated extracellular signal-regulated kinases (ERK1/2) is epithelial cell-specific in an inhalation model of asbestosis.
Cummins AB, Palmer C, Mossman BT, Taatjes DJ. Cummins AB, et al. Am J Pathol. 2003 Mar;162(3):713-20. doi: 10.1016/S0002-9440(10)63867-9. Am J Pathol. 2003. PMID: 12598305 Free PMC article. - Analyzing the genes and peptide growth factors expressed in lung cells in vivo consequent to asbestos exposure and in vitro.
Brody AR, Liu JY, Brass D, Corti M. Brody AR, et al. Environ Health Perspect. 1997 Sep;105 Suppl 5(Suppl 5):1165-71. doi: 10.1289/ehp.97105s51165. Environ Health Perspect. 1997. PMID: 9400718 Free PMC article. Review. - Cell signaling and transcription factor activation by asbestos in lung injury and disease.
Shukla A, Ramos-Nino M, Mossman B. Shukla A, et al. Int J Biochem Cell Biol. 2003 Aug;35(8):1198-209. doi: 10.1016/s1357-2725(02)00315-1. Int J Biochem Cell Biol. 2003. PMID: 12757757 Review.
Cited by
- Activated cAMP response element binding protein is overexpressed in human mesotheliomas and inhibits apoptosis.
Shukla A, Bosenberg MW, MacPherson MB, Butnor KJ, Heintz NH, Pass HI, Carbone M, Testa JR, Mossman BT. Shukla A, et al. Am J Pathol. 2009 Nov;175(5):2197-206. doi: 10.2353/ajpath.2009.090400. Epub 2009 Oct 8. Am J Pathol. 2009. PMID: 19815709 Free PMC article. - PKCδ/midkine pathway drives hypoxia-induced proliferation and differentiation of human lung epithelial cells.
Zhang H, Okamoto M, Panzhinskiy E, Zawada WM, Das M. Zhang H, et al. Am J Physiol Cell Physiol. 2014 Apr 1;306(7):C648-58. doi: 10.1152/ajpcell.00351.2013. Epub 2014 Feb 5. Am J Physiol Cell Physiol. 2014. PMID: 24500281 Free PMC article. - Gene expression profiles in asbestos-exposed epithelial and mesothelial lung cell lines.
Nymark P, Lindholm PM, Korpela MV, Lahti L, Ruosaari S, Kaski S, Hollmén J, Anttila S, Kinnula VL, Knuutila S. Nymark P, et al. BMC Genomics. 2007 Mar 1;8:62. doi: 10.1186/1471-2164-8-62. BMC Genomics. 2007. PMID: 17331233 Free PMC article. - Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein.
Park JA, Crews AL, Lampe WR, Fang S, Park J, Adler KB. Park JA, et al. Am J Pathol. 2007 Dec;171(6):1822-30. doi: 10.2353/ajpath.2007.070318. Epub 2007 Nov 30. Am J Pathol. 2007. PMID: 18055557 Free PMC article. - The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis.
Kim SJ, Cheresh P, Jablonski RP, Williams DB, Kamp DW. Kim SJ, et al. Int J Mol Sci. 2015 Sep 7;16(9):21486-519. doi: 10.3390/ijms160921486. Int J Mol Sci. 2015. PMID: 26370974 Free PMC article. Review.
References
- Mossman BT, Gee JB: Asbestos-related diseases. N Engl J Med 1989, 320:1721-1730 - PubMed
- Mossman BT, Bignon J, Corn M, Seaton A, Gee JB: Asbestos: scientific developments and implications for public policy. Science 1990, 247:294-301 - PubMed
- Mossman BT, Kamp DW, Weitzman SA: Mechanisms of carcinogenesis and clinical features of asbestos-associated cancers. Cancer Invest 1996, 14:466-480 - PubMed
- Mossman BT, Churg A: Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998, 157:1666-1680 - PubMed
- Perderiset M, Marsh JP, Mossman BT: Activation of protein kinase C by crocidolite asbestos in hamster tracheal epithelial cells. Carcinogenesis 1991, 12:1499-1502 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous