The mechanism of inhibition of Ran-dependent nuclear transport by cellular ATP depletion - PubMed (original) (raw)
Comparative Study
. 2002 Jun 10;157(6):963-74.
doi: 10.1083/jcb.200111077. Epub 2002 Jun 10.
Affiliations
- PMID: 12058015
- PMCID: PMC2174045
- DOI: 10.1083/jcb.200111077
Comparative Study
The mechanism of inhibition of Ran-dependent nuclear transport by cellular ATP depletion
Eric D Schwoebel et al. J Cell Biol. 2002.
Abstract
Rran-dependent nuclear transport requires a nuclear pool of RanGTP both for the assembly of export complexes and the disassembly of import complexes. Accordingly, in order for these processes to proceed, Ran-dependent nuclear import and export assays in vitro require the addition of GTP to produce RanGTP. Notably, no ATP requirement can be detected for these transport processes in vitro. But in vivo, when cells are depleted of ATP by the addition of sodium azide and 2-deoxyglucose to block ATP production by oxidative phosphorylation and glycolysis, respectively, Ran-dependent nuclear import and export are rapidly inhibited. This raised the question of whether there is an ATP requirement for these nuclear transport pathways in an intact cell that has remained undetected in vitro. Here we report that the free (but not total) GTP concentration rapidly drops to an undetectable level upon ATP depletion as does the availability of RanGTP. Our conclusion is that the inhibition of Ran-dependent nuclear transport observed upon ATP depletion in vivo results from a shortage of RanGTP rather than the inhibition of some ATP-dependent process.
Figures
Figure 1.
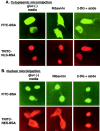
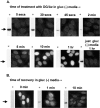
ATP depletion of cells with 2-deoxyglucose/Na azide inhibits both nuclear import and export, whereas GTP depletion with ribavirin does not. HeLa cells were incubated in gluc− media (2 h), gluc− media containing ribavirin (2 h), or gluc− media containing 2-deoxyglucose/azide (20 min). Cells were injected in the cytoplasm (A) with FITC–BSA marker (1.25 mg/ml) and the nuclear import reporter TRITC–NLS-BSA (1.3 mg/ml), or in the nucleus (B) with the marker and the nuclear export reporter TRITC–NES-BSA (4.5 mg/ml). After injection, the cells were incubated for an additional 30 min in the indicated medium followed by fixation and fluorescence microscopy.
Figure 2.
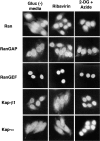
The cellular localization of nuclear transport factors after 2-deoxyglucose/sodium azide or ribavirin treatment. Cells were treated with control gluc− media for 2 h, gluc− media containing ribavirin for 2 h, or gluc− media containing 2-deoxyglucose/sodium azide for 1 h. The cells were fixed and probed with antibodies against the indicated nuclear transport factor, followed by fluorescently labeled second antibody. Note that Kap-α accumulates in the nuclei of 2-deoxyglucose/azide-treated cells, whereas the distribution of the other transport factors remains essentially unchanged.
Figure 3.
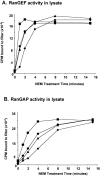
NEM treatment of HeLa cellular lysates inhibits both RanGEF and RanGAP activity. Cellular lysates were prepared as described in the Materials and methods and treated with either (♦) 10, (▴) 20, (•) 40, or (▪) 60 mM NEM for the indicated period of time, quenched with excess DTT, and measured as follows: (A) measurement of RanGEF activity in HeLa cell extracts after NEM treatment; (B) measurement of RanGAP activity in HeLa cell extracts after NEM treatment. Both RanGAP and GEF activity were measured as described in the Materials and methods.
Figure 4.
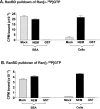
Isolation of RanGTP with a RanBD fused to GST. (A and B) NEM treatment of cellular lysate allows the recovery of RanGTP. Either cellular lysates or a solution of BSA were spiked with recombinant Ran loaded with either (A) [α-32P]GTP or (B) [γ-32P]GTP before pulldown of RanGTP with GST–RanBD. The radiolabeled Ran and NEM were added together with 2 mM GDP-βS upon cell lysis as described in the Materials and methods. Samples were either NEM treated to abolish RanGAP and GEF activity (100 mM NEM for 20 min) before quenching with 200 mM DTT, or mock treated in which the order of addition of NEM and DTT was reversed. After gel filtration to remove excess NEM and DTT, RanGTP was isolated in a pulldown with GST–RanBD and glutathione-agarose. In the indicated samples, GST was substituted for the GST–RanBD; these samples were mock treated. Shown are the CPM of radiolabeled Ran associated with the GST–RanBD glutathione-agarose beads after washing. (C) No free RanGTP can be detected in HeLa cells treated with 2-deoxyglucose/azide. HeLa cells were either untreated (lanes 1 and 6–8), incubated in gluc− media for either 1 (lane 2) or 2 h (lane 4), incubated with 2-deoxyglucose/azide in gluc− media for 1 h (lane 3), or incubated with ribavirin in gluc− media for 2 h (lane 5). After treatment, cells were scraped in buffer A containing 0.1% Triton X-100 and were either NEM treated as above to abolish RanGAP and GEF activity (lanes 1–7) or mock treated (lane 8). The unlabeled nucleotides added upon cellular lysis were 2 mM GDP-βS (lanes 1–5 and 8), GMP-PNP (lane 6), or GTP (lane 7). After NEM or mock treatment, RanGTP was isolated from the soluble cytosolic extract by incubation with GST–RanBD and glutathione-agarose. Bound protein was solubilized with SDS-PAGE sample buffer, separated on a 15% gel, transferred to nitrocellulose, and immunoblotted with an anti-Ran antibody. Note in particular the absence of detectable RanGTP in cells treated with 2-deoxyglucose/azide (lane 3). (D) Immunoblot of total cell extracts shows that the amount of total cellular Ran doesn't change after these treatments.
Figure 4.
Isolation of RanGTP with a RanBD fused to GST. (A and B) NEM treatment of cellular lysate allows the recovery of RanGTP. Either cellular lysates or a solution of BSA were spiked with recombinant Ran loaded with either (A) [α-32P]GTP or (B) [γ-32P]GTP before pulldown of RanGTP with GST–RanBD. The radiolabeled Ran and NEM were added together with 2 mM GDP-βS upon cell lysis as described in the Materials and methods. Samples were either NEM treated to abolish RanGAP and GEF activity (100 mM NEM for 20 min) before quenching with 200 mM DTT, or mock treated in which the order of addition of NEM and DTT was reversed. After gel filtration to remove excess NEM and DTT, RanGTP was isolated in a pulldown with GST–RanBD and glutathione-agarose. In the indicated samples, GST was substituted for the GST–RanBD; these samples were mock treated. Shown are the CPM of radiolabeled Ran associated with the GST–RanBD glutathione-agarose beads after washing. (C) No free RanGTP can be detected in HeLa cells treated with 2-deoxyglucose/azide. HeLa cells were either untreated (lanes 1 and 6–8), incubated in gluc− media for either 1 (lane 2) or 2 h (lane 4), incubated with 2-deoxyglucose/azide in gluc− media for 1 h (lane 3), or incubated with ribavirin in gluc− media for 2 h (lane 5). After treatment, cells were scraped in buffer A containing 0.1% Triton X-100 and were either NEM treated as above to abolish RanGAP and GEF activity (lanes 1–7) or mock treated (lane 8). The unlabeled nucleotides added upon cellular lysis were 2 mM GDP-βS (lanes 1–5 and 8), GMP-PNP (lane 6), or GTP (lane 7). After NEM or mock treatment, RanGTP was isolated from the soluble cytosolic extract by incubation with GST–RanBD and glutathione-agarose. Bound protein was solubilized with SDS-PAGE sample buffer, separated on a 15% gel, transferred to nitrocellulose, and immunoblotted with an anti-Ran antibody. Note in particular the absence of detectable RanGTP in cells treated with 2-deoxyglucose/azide (lane 3). (D) Immunoblot of total cell extracts shows that the amount of total cellular Ran doesn't change after these treatments.
Figure 5.
A time course of 2-deoxyglucose/azide treatment and recovery comparing the availability of RanGTP with the nuclear buildup of Kap-α. For each time point in A and B, the cellular localization of Kap-α as determined by indirect immunofluorescence microscopy (performed as in Fig. 2) was compared with the RanGTP from NEM-treated cellular extracts that bound to the RanBD (performed as in Fig. 4 C, lanes 2 and 3, arrow). (A) HeLa cells were incubated for the indicated time at 37°C in gluc− media containing 2-deoxyglucose/azide. (B) After treatment in 2-deoxyglucose/azide for 1 h, the cells were washed once in prewarmed gluc− media, and then transferred to prewarmed gluc− media and incubated at 37°C for the indicated time. To emphasize the heterogeneity in Kap-α nuclear accumulation at longer time points, the same relatively short camera exposure time was used for all of these micrographs.
Figure 6.
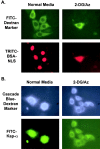
**The entry of microinjected FITC–Kap-**α into the nucleus is inhibited by 2-deoxyglucose/azide treatment. HeLa cells were incubated in either normal media or gluc− media containing 2-deoxyglucose/azide for 20 min. They were then microinjected into the cytoplasm with either (A) TRITC–NLS-BSA (9.1 mg/ml) and a FITC 70-kD dextran marker (6.7 mg/ml) or (B) FITC–Kap-α (1.7 mg/ml) and a Cascade blue 70-kD dextran marker (0.9 mg/ml). After injection (which took ∼10 min), the cells were incubated for an additional 30 min in the treatment media before fixation.
Similar articles
- The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus.
Izaurralde E, Kutay U, von Kobbe C, Mattaj IW, Görlich D. Izaurralde E, et al. EMBO J. 1997 Nov 3;16(21):6535-47. doi: 10.1093/emboj/16.21.6535. EMBO J. 1997. PMID: 9351834 Free PMC article. - NTF2 mediates nuclear import of Ran.
Ribbeck K, Lipowsky G, Kent HM, Stewart M, Görlich D. Ribbeck K, et al. EMBO J. 1998 Nov 16;17(22):6587-98. doi: 10.1093/emboj/17.22.6587. EMBO J. 1998. PMID: 9822603 Free PMC article. - Protein export from the nucleus requires the GTPase Ran and GTP hydrolysis.
Moroianu J, Blobel G. Moroianu J, et al. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4318-22. doi: 10.1073/pnas.92.10.4318. Proc Natl Acad Sci U S A. 1995. PMID: 7753805 Free PMC article. - Two-way trafficking with Ran.
Melchior F, Gerace L. Melchior F, et al. Trends Cell Biol. 1998 May;8(5):175-9. doi: 10.1016/s0962-8924(98)01252-5. Trends Cell Biol. 1998. PMID: 9695834 Review. - Transport of macromolecules between the nucleus and the cytoplasm.
Izaurralde E, Adam S. Izaurralde E, et al. RNA. 1998 Apr;4(4):351-64. RNA. 1998. PMID: 9630243 Free PMC article. Review.
Cited by
- Mechanism of exportin retention in the cell nucleus.
Kapinos LE, Kalita J, Kassianidou E, Rencurel C, Lim RYH. Kapinos LE, et al. J Cell Biol. 2024 Feb 5;223(2):e202306094. doi: 10.1083/jcb.202306094. Epub 2024 Jan 19. J Cell Biol. 2024. PMID: 38241019 Free PMC article. - Cellular stresses induce the nuclear accumulation of importin alpha and cause a conventional nuclear import block.
Miyamoto Y, Saiwaki T, Yamashita J, Yasuda Y, Kotera I, Shibata S, Shigeta M, Hiraoka Y, Haraguchi T, Yoneda Y. Miyamoto Y, et al. J Cell Biol. 2004 Jun 7;165(5):617-23. doi: 10.1083/jcb.200312008. J Cell Biol. 2004. PMID: 15184398 Free PMC article. - Late stages of the synchronized macrophage fusion in osteoclast formation depend on dynamin.
Verma SK, Leikina E, Melikov K, Chernomordik LV. Verma SK, et al. Biochem J. 2014 Dec 15;464(3):293-300. doi: 10.1042/BJ20141233. Biochem J. 2014. PMID: 25336256 Free PMC article. - Molecular Coevolution of Nuclear and Nucleolar Localization Signals inside the Basic Domain of HIV-1 Tat.
Kurnaeva MA, Zalevsky AO, Arifulin EA, Lisitsyna OM, Tvorogova AV, Shubina MY, Bourenkov GP, Tikhomirova MA, Potashnikova DM, Kachalova AI, Musinova YR, Golovin AV, Vassetzky YS, Sheval EV. Kurnaeva MA, et al. J Virol. 2022 Jan 12;96(1):e0150521. doi: 10.1128/JVI.01505-21. Epub 2021 Oct 6. J Virol. 2022. PMID: 34613791 Free PMC article. - HIV-1 integrase is capable of targeting DNA to the nucleus via an importin alpha/beta-dependent mechanism.
Hearps AC, Jans DA. Hearps AC, et al. Biochem J. 2006 Sep 15;398(3):475-84. doi: 10.1042/BJ20060466. Biochem J. 2006. PMID: 16716146 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous