Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand - PubMed (original) (raw)
. 2002 May 31;109(5):625-37.
doi: 10.1016/s0092-8674(02)00754-7.
Koichi Hattori, Sergio Dias, Matthias Friedrich, Barbara Ferris, Neil R Hackett, Ronald G Crystal, Peter Besmer, David Lyden, Malcolm A S Moore, Zena Werb, Shahin Rafii
Affiliations
- PMID: 12062105
- PMCID: PMC2826110
- DOI: 10.1016/s0092-8674(02)00754-7
Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand
Beate Heissig et al. Cell. 2002.
Abstract
Stem cells within the bone marrow (BM) exist in a quiescent state or are instructed to differentiate and mobilize to circulation following specific signals. Matrix metalloproteinase-9 (MMP-9), induced in BM cells, releases soluble Kit-ligand (sKitL), permitting the transfer of endothelial and hematopoietic stem cells (HSCs) from the quiescent to proliferative niche. BM ablation induces SDF-1, which upregulates MMP-9 expression, and causes shedding of sKitL and recruitment of c-Kit+ stem/progenitors. In MMP-9-/- mice, release of sKitL and HSC motility are impaired, resulting in failure of hematopoietic recovery and increased mortality, while exogenous sKitL restores hematopoiesis and survival after BM ablation. Release of sKitL by MMP-9 enables BM repopulating cells to translocate to a permissive vascular niche favoring differentiation and reconstitution of the stem/progenitor cell pool.
Figures
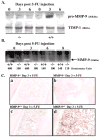
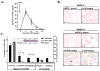
Figure 1. MMP-9 Is Induced in BM Cells after BM Ablation
(A and B) MMP-9−/− and MMP-9+/+ mice received a single dose of 5-FU i.v. BM cells obtained at different time points after 5-FU injection were cultured in serum-free medium overnight. BM cell supernatants were assayed for pro-MMP-9 by Western blot (A) and active MMP-9 by gelatin zymography (B). Molecular weight (kDa) are shown (n = 5/group). (C) Immunohistochemistry of BM sections three days after 5-FU injection for pro-MMP-9, which shows brown staining of stromal and hematopoietic elements in MMP-9+/+ mice (c and d), but not in MMP-9−/− mice (a and b); magnification ×100, (a and c) and ×400 (b and d).
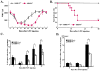
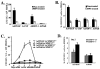
Figure 2. Delayed Hematopoietic Recovery and Increased Mortality in MMP-9−/− Mice after Myelosuppression
(A and B) MMP-9−/− and MMP-9+/+ mice (n = 16) received a single i.v. dose of 5-FU. (A) WBC counts were quantified by a Neubauer chamber. (B) Survival of 5-FU-treated mice was assessed daily (n = 16). (C and D) Mice treated with 5-FU were sacrificed at different time points. Percentage/total number of Sca-1+ (n = 5) and Lin−Sca-1+c-Kit+ (n = 7) cells, isolated by a combination of magnetic cell isolation (MACS) and flow cytometry (FACS), in S phase was determined for DNA content after propidium iodide staining. The total number of Sca-1+ cells in S phase was higher in MMP-9+/+ as compared to MMP-9−/− mice (2.2 ± 0.05 versus 1.0 ± 0.03 × 105/femur on day 6 and 11.5 ± 0.3 versus 2.1 ± 0.04 × 105/femur on day 10, respectively). All values are given as mean ± SEM. *p < 0.01, **p < 0.001.
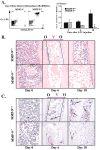
Figure 3. Recruitment and Differentiation of Hematopoietic Cells Are Impaired in MMP-9−/− Mice
MMP-9−/− and MMP-9+/+ mice were treated with 5-FU and the hematopoietic recovery and the frequency and distribution of myeloid and megakaryocytic precursor cells was evaluated by FACS (A) or immunohistochemistry (B and C). (A) BM cells obtained from either MMP-9−/− or MMP-9+/+ mice were stained for the myeloid markers CD11b-FITC and Gr-1-PE and analyzed by FACS (left panel). Absolute number of CD11b+/Gr-1+ BM cells per femur was calculated at different time points (right panel, n = 6, *p < 0.01). (B) H&E staining of femurs from mice after 5-FU treatment. Hematopoietic cell clusters can be detected in close contact to osteoblasts (Osteoblastic zone, O) in the early phase of BM recovery. Over time, abundant clusters of proliferating hematopoietic cells are detected both in the osteoblastic zone and in the vascular-enriched zone (Vascular zone, V) in wild-type animals. In contrast, there is a striking paucity of hematopoietic cell clusters in the osteoblastic and the vascular zone in 5-FU-treated MMP-9−/− mice. (C) vWF staining (brown) of femurs at different time points following 5-FU treatment. vWF positive megakaryocytes increase during BM recovery in MMP-9+/+ mice, but not in MMP-9−/− mice (arrows; magnification ×100).
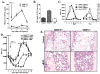
Figure 4. MMP-9 Mediated Release of sKitL Enhances Hematopoietic Reconstitution
(A) MMP-9−/− and MMP-9+/+ mice were injected i.v. with a single dose of 5-FU and the plasma obtained from peripheral blood (PB) was assayed for sKitL by ELISA (p< 0.05, n = 6/group). (B) Confluent MS-5 murine stromal cells, which express mKitL, were treated with recombinant active MMP-9 or the MPI CGS 27023A for 24 hr.*p< 0.001. (C) MMP-9−/− and MMP-9+/+ mice were injected i.v. on day 0 with a single dose of Ad vector encoding for sKitL (AdsKitL) or no transgene (AdNull). PB was taken at indicated days (n = 6/group). Injection of AdsKitL resulted in sKitL plasma levels of 5399 ± 50 and 5126 ± 102 pg/ml on day 5 in MMP-9−/− and MMP-9+/+ mice, respectively. PBMCs were stained for Sca-1 and c-Kit and analyzed by FACS. (D) MMP-9−/− and MMP-9+/+ mice were injected with recombinant sKitL from day 3–11 after 5-FU therapy (n = 10/group). WBC counts were determined at indicated time points. (E) H&E staining of BM sections 4 days after 5-FU marrow suppression in MMP-9−/− and MMP-9+/+ mice without (control) and with sKitL Magnification × 200.
Figure 5. Chemo/Cytokines Induce MMP-9 Expression in BM Hematopoietic Cells
(A) MMP-9+/+ mice were treated with a single dose of 5-FU. At indicated time points, plasma was analyzed for SDF-1 by ELISA (n = 6/time point). (B) MMP-9−/− and MMP-9+/+ mice received AdSDF-1, AdVEGF, and AdNull vector by a single i.v. injection. BM sections were stained for pro-MMP-9. MMP-9−/− mice treated with G-CSF served as negative control (a and b). BM sections after AdSDF-1 (c and d), AdVEGF (e), and G-CSF (f) in MMP-9+/+ mice. Magnification × 100 (a and c), × 400 (b, d–f). (C) Human CD34+ cells were plated in Matrigel-coated transwells. MPIs (5-phenyl-1,10-phenanthroline and CGS 27023A) or PBS were added to both chambers. The chemoattractant SDF-1 was added to the lower chamber. Data are shown as a percentage of migrated cells (black bar). Migrated stem cells assayed as absolute number of CAFC at week 5 (open bar) and of LTC-IC (hatched bar; n = 3, *p< 0.05 for the migration of cells treated with/without MPI toward SDF-1). Insert: Gelatin zymogram of culture supernatants from human CD34+ cells stimulated with/without SDF-1 or VEGF in serum-free medium. Supernatants from CD34+ cell cultures showed gelatinolytic activity for pro-MMP-9 (92 kDa).
Figure 6. Chemo/Cytokine-Induced HSC Mobilization Is Impaired in MMP-9−/− Mice
(A–C) MMP-9−/− and MMP-9+/+ mice were injected i.v. with a single dose of AdSDF-1, AdVEGF, and AdNull vector or s.c. with recombinant G-CSF from day 0–5 (n = 10 mice in each group). Elevated chemokine levels for SDF-1 and VEGF were achieved by adenoviral gene delivery of SDF-1 and VEGF ([A] and [B], bar graph insert). WBC counts were determined following AdSDF-1 (A), AdVEGF (B), and G-CSF treatment in MMP-9+/+ mice (C). (D) Mobilized PBMCs were plated in a colony assay. The number of mobilized progenitor cells (CFU-C) was determined (n = 10, *0.05, **p< 0.01) on day 5 (AdSDF-1), on day 3 (AdVEGF), and on day 5 (G-CSF). (E) PB of MMP-9−/− and MMP-9+/+ mice treated with or without G-CSF was obtained on day 5. PBMCs were transplanted into lethally irradiated syngeneic animals. Survival of transplanted recipients was monitored (n = 8/group, *p< 0.001). (F) Plasma of MMP-9+/+ and MMP-9−/− mice 5 days after G-CSF, AdNull, AdSDF-1, and AdVEGF injection and untreated controls was assayed for sKitL (n = 6/group, *p< 0.03).
Figure 7. Endothelial Cell Progenitor Mobilization Is Blocked in MMP-9−/− Mice
The number of circulating endothelial progenitor cells (CEPs), represented in in vitro cultures as colony forming units of endothelial cells (CFU-EC) were determined in the PB of MMP-9+/+ and MMP-9−/− mice three days after injection with AdVEGF, AdSDF-1, AdNull, and G-CSF in the presence or absence of MPI. (A and B) CEPs were quantified by the formation of CFU-EC and by VEGFR2+ cells detected by FACS. The increase in circulating CFU-EC in VEGF-treated animals correlated with the number of VEGFR2+ cells (*p < 0.05). (C and D) MMP-9−/− and MMP-9+/+ mice were injected i.v. with a single dose of AdVEGF and AdNull vector (n = 6/group, *p< 0.05). Mobilized PBMC were assayed for the number of CFU-EC in circulation (C) and the number of VEGFR2+ cells in the BM on day 6 after adenoviral injection (D).
Figure 8. Functional Anatomy and Recruitment of c-Kit+ Stem and Progenitor Cells Is Dictated by MMP-9 Mediated Release of sKitL
Under steady-state conditions quiescent c-Kit+ HSCs and CEPs reside in a niche in close contact with stromal cells including osteoblasts. Membrane-bound cytokines, such as mKitL not only convey survival signals, but also support the adhesion of stem cells to the stroma. BM ablation or chemokine/cytokine administration induces upregulation of MMP-9 resulting in the release of sKitL sKitL confers signals that enhances mobility of VEGFR2+ endothelial progenitors (CEPs) and Lin−Sca-1+c-Kit+ repopulating cells, translocating them into a vascular-enriched niche favoring differentiation and mobilization to the peripheral circulation.
Similar articles
- Angiogenic factors reconstitute hematopoiesis by recruiting stem cells from bone marrow microenvironment.
Rafii S, Avecilla S, Shmelkov S, Shido K, Tejada R, Moore MA, Heissig B, Hattori K. Rafii S, et al. Ann N Y Acad Sci. 2003 May;996:49-60. doi: 10.1111/j.1749-6632.2003.tb03232.x. Ann N Y Acad Sci. 2003. PMID: 12799282 - The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1.
Hattori K, Heissig B, Rafii S. Hattori K, et al. Leuk Lymphoma. 2003 Apr;44(4):575-82. doi: 10.1080/1042819021000037985. Leuk Lymphoma. 2003. PMID: 12769333 - Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment.
Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S. Hattori K, et al. Nat Med. 2002 Aug;8(8):841-9. doi: 10.1038/nm740. Epub 2002 Jul 1. Nat Med. 2002. PMID: 12091880 Free PMC article. - Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties.
Pelus LM, Fukuda S. Pelus LM, et al. Exp Hematol. 2006 Aug;34(8):1010-20. doi: 10.1016/j.exphem.2006.04.004. Exp Hematol. 2006. PMID: 16863907 Review.
Cited by
- The crosstalk between lung cancer and the bone marrow niche fuels emergency myelopoiesis.
Calderon-Espinosa E, De Ridder K, Benoot T, Jansen Y, Vanhonacker D, Heestermans R, De Becker A, Van Riet I, Decoster L, Goyvaerts C. Calderon-Espinosa E, et al. Front Immunol. 2024 Aug 1;15:1397469. doi: 10.3389/fimmu.2024.1397469. eCollection 2024. Front Immunol. 2024. PMID: 39148724 Free PMC article. Review. - Dynamics of Endothelial Cell Diversity and Plasticity in Health and Disease.
Larionov A, Hammer CM, Fiedler K, Filgueira L. Larionov A, et al. Cells. 2024 Jul 29;13(15):1276. doi: 10.3390/cells13151276. Cells. 2024. PMID: 39120307 Free PMC article. Review. - Hematopoietic Stem Cells and Their Niche in Bone Marrow.
Kwon M, Kim BS, Yoon S, Oh SO, Lee D. Kwon M, et al. Int J Mol Sci. 2024 Jun 21;25(13):6837. doi: 10.3390/ijms25136837. Int J Mol Sci. 2024. PMID: 38999948 Free PMC article. Review. - Role of Neurotransmitters in Steady State Hematopoiesis, Aging, and Leukemia.
Beeraka NM, Basappa B, Nikolenko VN, Mahesh PA. Beeraka NM, et al. Stem Cell Rev Rep. 2024 Jul 8. doi: 10.1007/s12015-024-10761-z. Online ahead of print. Stem Cell Rev Rep. 2024. PMID: 38976142 Review. - Granulocyte Colony Stimulating Factor-Mobilized Peripheral Blood Mononuclear Cells: An Alternative Cellular Source for Chimeric Antigen Receptor Therapy.
Ballesteros-Ribelles A, Millán-López A, Carmona-Luque M, Herrera C. Ballesteros-Ribelles A, et al. Int J Mol Sci. 2024 May 25;25(11):5769. doi: 10.3390/ijms25115769. Int J Mol Sci. 2024. PMID: 38891957 Free PMC article. Review.
References
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. - PubMed
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR046238-06A2/AR/NIAMS NIH HHS/United States
- HL-66592/HL/NHLBI NIH HHS/United States
- HL-67839/HL/NHLBI NIH HHS/United States
- HL-61849/HL/NHLBI NIH HHS/United States
- P01 CA072006-07/CA/NCI NIH HHS/United States
- P01 HL067839/HL/NHLBI NIH HHS/United States
- CA 72006/CA/NCI NIH HHS/United States
- AR 46238/AR/NIAMS NIH HHS/United States
- R01 HL061849/HL/NHLBI NIH HHS/United States
- CA 75072/CA/NCI NIH HHS/United States
- R01 CA075072-03/CA/NCI NIH HHS/United States
- R01 AR046238/AR/NIAMS NIH HHS/United States
- P01 CA072006/CA/NCI NIH HHS/United States
- HL-58707/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous