A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis - PubMed (original) (raw)
A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis
Roland Wedlich-Söldner et al. EMBO J. 2002.
Abstract
In Ustilago maydis, bidirectional transport of early endosomes is microtubule dependent and supports growth and cell separation. During early budding, endosomes accumulate at putative microtubule organizers within the bud, whereas in medium-budded cells, endosome clusters appear at the growing ends of microtubules at the distal cell pole. This suggests that motors of opposing transport direction organize endosomes in budding cells. Here we set out to identify these motors and elucidate the molecular mechanism of endosome reorganization. By PCR we isolated kin3, which encodes an UNC-104/KIF1-like kinesin from U.maydis. Recombinant Kin3 binds microtubules and has ATPase activity. Kin3-green fluorescent protein moves along microtubules in vivo, accumulates at sites of growth and localizes to endosomes. Deletion of kin3 reduces endosome motility to approximately 33%, and abolishes endosome clustering at the distal cell pole and at septa. This results in a transition from bipolar to monopolar budding and cell separation defects. Double mutant analysis indicates that the remaining motility in Deltakin3-mutants depends on dynein, and that dynein and Kin3 counteract on the endosomes to arrange them at opposing cell poles.
Figures
Fig. 1. Sequence analysis of Kin3. (A) Domain organization of Kin3 and close relatives. Kin3 contains all domains typical for members of the UNC-104/KIF1 family. This includes the N-terminal motor domain (red), an FHA domain (yellow), a PH domain (blue) and short coiled-coil regions (green). (B) Dot-plot comparison of the predicted amino acid sequence of Kin3 versus MmKIF1A. Note that both motors share sequence similarity along the entire length of the molecule. (C) Nearest-neighbor dendrogram of potential kinesin organelle transporters. Kin3 groups with members of the UNC-104/KIF1 family. The dendogram is based on a distance matrix-based neighbor-joining analysis of the kinesin motor domain. Statistical support for this tree was obtained by bootstrapping (neighbor joining and parsimony, 1000 resamplings each) or Quartet Puzzling. Solid circles indicate a bootstrap and QP support of >80%, while open circles indicate 60–80% support. Accession numbers are given. The bar reflects 0.1 changes per amino acid.
Fig. 2. MT affinity, ATPase activity and in vivo motility of Kin3. (A) MT affinity of recombinant Kin3 heads. After ATP depletion, bacterial expressed Kin3 motor domains bound to taxol-stabilized MTs, and were partially released by MgATP (S5), while some protein was sedimented with MTs (P6). Kin31–358, amino acids 1–358; Kin31–431, amino acids 1–431 of Kin3 (arrows). (B) His-tagged Kin3 was affinity purified (B1) and ATPase activity, shown as NADH reduction per time in the presence of increasing tubulin concentrations, was measured (B2). Note that the neck region (amino acids 358–431) is required for MT stimulation of truncated motor domain. (C) Movement of Kin3–GFP. The Kin3–GFP fusion protein (arrow) moves rapidly in a bidirectional fashion. Time between frames is 500 ms. Bar: 3 µm. (D) Co-localization of Kin3–YFP- and CFP–Tub1-marked microtubules. MTs are running through the length of an unbudded cell (D1). A series of images of Kin3–YFP (green, arrows) was taken and merged with a single image of CFP–Tub1 (red). Movement of a large accumulation of Kin3–YFP (D2), as well as of small Kin3–YFP-labeled dots (D3) along MTs. Time between frames is 700 ms. Bar: 2 µm (D1) and 1 µm (D2 and D3). A movie for this figure is available as Supplementary data at The EMBO Journal Online.
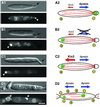
Fig. 3. Early endosomes and Kin3. (A) Overview of the organization of EEs and MTs during the cell cycle of U.maydis. Unbudded cells show bidirectional traffic of EEs (green spheres) along antipolar MTs (A1; orientation of MTs indicated by ‘+’ and ‘–’; motility indicated by red arrows). Polar budding is accompanied by the formation of an EE cluster at the minus-end of the MTs (A2), which is due to a significant net movement towards the bud (bold red arrow). A similar situation is found in medium-budded cells that contain EE clusters at their rear cell pole (A3). No motility occurs during mitosis (A4). Finally, septum formation is accompanied by one and, in later stages, two endosomal clusters at the septa (A5). During this stage, antipolar MT bundles are found. MT polarity is based on in vivo observation of dynamics of GFP-MTs (Steinberg et al., 2001), and EE behavior is described in Wedlich-Söldner et al. (2000). (B) Cell cycle-dependent accumulation of Kin3–GFP fusion protein under the control of its native promoter (strain RWS16). Kin3–GFP localizes to small buds during early budding (B1). In medium-budded cells, a strong signal of Kin3–GFP appears at the distal cell pole (B2), which finally locates to the region of septum formation (B3). Note that Kin3–GFP localization corresponds well with endosome rearrangement (A). Bar: 3 µm. (C) Co-localization of EEs and Kin3–GFP. In pulse–chase experiments, FM4-64 appears in EEs (C1) that also carry Kin3–GFP (C2; overlay in C3). Note that, occasionally, EEs do not co-localize with Kin3–GFP fusion protein. Bar: 3 µm.
Fig. 4. Quantitative analysis of endosome motility. (A) Percentage of moving EEs. In strain RWS4 (control), almost all Yup1–GFP-stained EEs showed movement. After deletion of kin3 (Δ_kin3_; RWS10) endosome motility decreased to ∼33%. This phenotype could be restored by expression of kin3 under the control of its native promoter (Δ_kin3_/p_kin3_; RWS11). Expression of a mutant allele of kin3 with a defect in the P-loop of the motor domain in Δ_kin3_ cells (Δ_kin3_/p_kin3_G105E; RWS19) resulted in a drastic decrease in the residual motility. High levels of Kin3 did not increase movement rates (crgkin3; RWS16). In a conditional dynein strain that was deleted for kin3 (Δ_kin3_Dynts; RWS14), movement of EEs at permissive temperature was similar to that of Δ_kin3_. However, shift to 33°C decreased EE motility to <1%. Bars represent mean percentage ± SD (n_ = 15 cells). (B) Velocity of EE motion. In the reference strain RWS4, EEs moved at ∼3 µm/s (control). This velocity decreased by >50% after deletion of kin3 (Δ_kin3). Both the expression of kin3 under the control of its native promoter (Δ_kin3_/p_kin3_), as well as overexpression (crgkin3), restored this defect. Overexpression of the point-mutated kin3 allele (Δ_kin3_/p_kin3_G105E) resulted in a clear decrease in velocity. In the temperature-sensitive dynein–Δ_kin3_ double mutant (Δ_kin3_Dynts) at 22°C, EEs move at the expected velocity (compare with Δ_kin3_). Almost all motility was abolished at 33°C, and the velocity was therefore not determined (n.d.). Bars represent mean velocities ± SD (n = 20 EEs, and 7 EEs for Δ_kin3_/p_kin3_G105E).
Fig. 5. Motion of EEs in the kin3 deletion strain RWS13. (A–C) Co-expression of a GFP–Tub1 fusion protein and Yup1–GFP in a Δ_kin3_ strain allowed us to analyze the role of MTs in the remaining motility of EEs. Brightly stained EE clusters move along MTs (A; arrows mark the MT). In the absence of Kin3, they were located at the ends of MTs. In all cases (n = 31), MTs rapidly shortened towards these cluster (B; end of MT marked by asterisk), suggesting that EEs are located at the minus-ends (labeled ‘+’ and ‘–’). Consistent with this, MT elongation was found to be directed away from the BSDs (C; ends of MTs marked by asterisks, orientation indicated by ‘+’ and ‘–’). Time between frames is 1.4 s. Bar: 2 µm. (D) Endosome organization in the temperature-sensitive dynein–Δ_kin3_ double mutant (RWS14). After 2 h at 33°C, endosome clusters (arrowheads) are smaller or even absent from the cell (D1; right cell). Note that cells have a separation defect due to the deletion of kin3. In these structures, almost no EE motility was found (D2, D3; time in seconds is given in the bottom right corner). Bars: 3 µm. (E) Expression of a dominant-negative kin3 mutant allele that is described to result in a rigid binding of the motor to the MT leads to ‘pearl-string’-like arrangement of EEs (arrows) and abolished almost all motion (compare E1 and E2). Bar: 2 µm; time in seconds is given in the bottom right corner. Movies are available as Supplementary data.
Fig. 6. Kin3 and dynein activity and endosome accumulation in small-budded cells. (A) In small-budded cells of control strain RWS4, accumulations of EEs are located within the bud (A1; arrow indicates bud; arrowhead marks BSD), and only ∼4% of the cells contain the BSD at the distal pole. Previous studies on the MT dynamics suggested that the minus-ends of MTs are located in the bud (Steinberg et al., 2001), indicating that cytoplasmic dynein moves EEs towards the growth region. EE motility was found to be bidirectional, indicating that Kin3 is also active (A2; MTs indicated by red lines, MT orientation marked by ‘+’ and ‘–’, and motor activity and direction indicated by arrows). (B) Heat inactivation of Dyn2ts in the conditional mutant strain RWS15 for 30–60 min shifted EEs to the distal cell pole (B1; arrow indicates bud; arrowhead marks BSD). This localization is in contrast to control cells and is most likely due to an alteration in the balance of Kin3 and dynein activity (B2). (C) High levels of Kin3 also result in strong accumulations of EEs at the distal cell pole of small-budded cells (C1; arrow indicates bud; arrowhead marks BSD). This reorganization is most likely the result of a disturbed balance between Kin3 and dynein (C2). (D) Deletion of kin3 led to randomly positioned EE accumulations (D1; arrowhead marks BSD). Δ_kin3_ cells fail to separate and have a MT organization that is typical for unbudded cells (compare with Figure 3A5). Endosome clusters are located at the minus-ends of these MTs, which is most likely due to the activity of dynein. Occasionally, bidirectional motility was observed, which occurred exclusively along bundles, suggesting that this motility is also dynein based (D2). Bar: 2 µm. Movies are available as Supplementary data.
Fig. 7. Morphology and colony phenotype of a Δ_kin3_ mutant strain. (A) In contrast to wild-type cells (small image at bottom right), the Δ_kin3_ strain forms tree-like aggregates in liquid culture. This phenotype is most likely due to a cell separation defect in combination with a mono-polar budding pattern. The order of bud formation is indicated by white numbers. Bar: 10 µm. (B) Calcofluor staining showed that septa are formed, indicating that deletion of kin3 affects late steps of cell separation. Bar: 2 µm. (C) Morphology of RWS18 leads to ring-like colonies on agar plates. Bar: 0.4 mm.
Fig. 8. Distribution of chitin and bud-site selection during the cell cycle of U.maydis. (A) Chitin was stained with WGA in strain FB2. Chitin accumulates strongly at the bud scars in all stages of the cell cycle (arrow in A1–A7). The new bud emerges at the cell pole opposite the bud scar (A2, A3), and chitin localizes to the tip and to the neck region (arrowheads in A3). In cells with larger buds, the chitin ring at the neck becomes more faint, while the tip still carries chitin (arrowhead in A4). Subsequently, chitin appears at the septa (arrowhead in A5), and two bud scars remain after cell separation (pair of arrows in A6). The next appears next to the old bud scar (arrowhead in A7), finally leading clusters of ring-like bud scars (small image in A7) at both ends of the cell. Bar: 3 µm. (B) In Δ_kin3_ cells, many bud scars accumulate at one cell pole (arrows). Bar: 3 µm.
Similar articles
- A dynein loading zone for retrograde endosome motility at microtubule plus-ends.
Lenz JH, Schuchardt I, Straube A, Steinberg G. Lenz JH, et al. EMBO J. 2006 Jun 7;25(11):2275-86. doi: 10.1038/sj.emboj.7601119. Epub 2006 May 11. EMBO J. 2006. PMID: 16688221 Free PMC article. - Dynein supports motility of endoplasmic reticulum in the fungus Ustilago maydis.
Wedlich-Söldner R, Schulz I, Straube A, Steinberg G. Wedlich-Söldner R, et al. Mol Biol Cell. 2002 Mar;13(3):965-77. doi: 10.1091/mbc.01-10-0475. Mol Biol Cell. 2002. PMID: 11907275 Free PMC article. - The role of microtubules in cellular organization and endocytosis in the plant pathogen Ustilago maydis.
Steinberg G, Fuchs U. Steinberg G, et al. J Microsc. 2004 May;214(Pt 2):114-23. doi: 10.1111/j.0022-2720.2004.01319.x. J Microsc. 2004. PMID: 15102060 Review. - Kinesin-3 and dynein mediate microtubule-dependent co-transport of mRNPs and endosomes.
Baumann S, Pohlmann T, Jungbluth M, Brachmann A, Feldbrügge M. Baumann S, et al. J Cell Sci. 2012 Jun 1;125(Pt 11):2740-52. doi: 10.1242/jcs.101212. Epub 2012 Feb 22. J Cell Sci. 2012. PMID: 22357951 - Cytoplasmic dynein and early endosome transport.
Xiang X, Qiu R, Yao X, Arst HN Jr, Peñalva MA, Zhang J. Xiang X, et al. Cell Mol Life Sci. 2015 Sep;72(17):3267-80. doi: 10.1007/s00018-015-1926-y. Epub 2015 May 23. Cell Mol Life Sci. 2015. PMID: 26001903 Free PMC article. Review.
Cited by
- Microtubule-dependent membrane dynamics in Ustilago maydis: Trafficking and function of Rab5a-positive endosomes.
Göhre V, Vollmeister E, Bölker M, Feldbrügge M. Göhre V, et al. Commun Integr Biol. 2012 Sep 1;5(5):485-90. doi: 10.4161/cib.21219. Commun Integr Biol. 2012. PMID: 23181166 Free PMC article. - Fluorescent markers of the endocytic pathway in Zymoseptoria tritici.
Kilaru S, Schuster M, Latz M, Guo M, Steinberg G. Kilaru S, et al. Fungal Genet Biol. 2015 Jun;79:150-7. doi: 10.1016/j.fgb.2015.03.019. Fungal Genet Biol. 2015. PMID: 26092801 Free PMC article. - Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures.
Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, Hansberg W, Herrera-Estrella A, Kämper J, Kück U, Mouriño-Pérez RR, Takeshita N, Fischer R. Riquelme M, et al. Microbiol Mol Biol Rev. 2018 Apr 11;82(2):e00068-17. doi: 10.1128/MMBR.00068-17. Print 2018 Jun. Microbiol Mol Biol Rev. 2018. PMID: 29643171 Free PMC article. Review. - The ESCRT regulator Did2 maintains the balance between long-distance endosomal transport and endocytic trafficking.
Haag C, Pohlmann T, Feldbrügge M. Haag C, et al. PLoS Genet. 2017 Apr 19;13(4):e1006734. doi: 10.1371/journal.pgen.1006734. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28422978 Free PMC article. - Roles of dynein and dynactin in early endosome dynamics revealed using automated tracking and global analysis.
Flores-Rodriguez N, Rogers SS, Kenwright DA, Waigh TA, Woodman PG, Allan VJ. Flores-Rodriguez N, et al. PLoS One. 2011;6(9):e24479. doi: 10.1371/journal.pone.0024479. Epub 2011 Sep 6. PLoS One. 2011. PMID: 21915335 Free PMC article.
References
- Apodaca G. (2001) Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic, 2, 149–159. - PubMed
- Bloom G.S. (1992) Motor proteins for cytoplasmic microtubules. Curr. Opin. Cell Biol., 4, 66–73. - PubMed
- Bloom G.S. (2001) The UNC-104/KIF1 family of kinesins. Curr. Opin. Cell Biol., 13, 36–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources