Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth - PubMed (original) (raw)
Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth
Sheng Wang et al. EMBO J. 2002.
Abstract
E2F transcription factors play a major role in controlling mammalian cell cycle progression. We recently reported that a potential tumor suppressor, prohibitin, which interacts with retinoblastoma protein (Rb), regulates E2F function and this activity correlates with its growth-suppressive activity. We show here that prohibitin recruits Brg-1/Brm to E2F-responsive promoters, and that this recruitment is required for the repression of E2F-mediated transcription by prohibitin. Expression of a dominant-negative Brg-1 or Brm releases prohibitin-mediated repression of E2F and relieves prohibitin-mediated growth suppression. Although prohibitin associates with, and recruits, Brg-1 and Brm independently of Rb, prohibitin/Brg-1/Brm-mediated transcriptional repression requires Rb. A viral oncoprotein, SV40 large T antigen, can reverse prohibitin-mediated suppression of E2F-mediated gene transcription, and targets prohibitin through interruption of the association between prohibitin and Brg-1/Brm without affecting the prohibitin-E2F interaction.
Figures
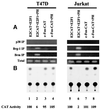
Fig. 1. Prohibitin-mediated transcription repression involves Brg-1 and Brm. (A) SW13 cells were transfected with the pSVECG CAT reporter, which is driven by the SV40 promoter and carries a GAL4-binding site along with pCR3.1GAL4DBD-prohibitin, pCGhBrm or both. The relative CAT activities were estimated by directly measuring the radioactivity recovered from the TLC plate and was calculated based on ‘pSVECG’ as ‘100’. The basal activity of the reporter was reduced an estimated 42 and 96% by co-transfection of prohibitin with 2 and 4 µg of Brm expression vector (lanes 4 and 5), while prohibitin alone (lane 2) or Brm alone (lane 3) did not cause strong repression. (B) Jurkat and T47D cells were transfected with the E2CAT reporter, the activity of which is induced by co-transfected E2F1 (lanes 1 and 2). Co-transfection of 2 µg of prohibitin completely repressed the CAT activity, while transfection of a 10-fold lower amount (0.2 µg) of prohibitin expression vector failed to repress the CAT activity. Co- transfection of increasing amounts of Brg-1 or Brm with 0.2 µg of prohibitin expression vector restored the repression of E2F-mediated CAT activity. The radioactivity of TLC was measured and the relative CAT activity was calculated based on ‘E2CAT + E2F1’ as ‘100’.
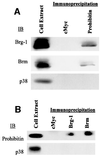
Fig. 2. Prohibitin interacts with Brg-1 and Brm in vivo. (A) Extracts from Jurkat cells were immunoprecipitated by c-Myc (control) or prohibitin antibodies followed by immunoblot analysis (IB) using Brg-1 or Brm antibodies. (B) The reverse immunoprecipitation (IP)/IB was carried out by using cMyc, Brg-1 or Brm antibodies for the IP and prohibitin antibody for the IB. p38 antibody failed to detect any band in the immunoprecipitates.
Fig. 3. Prohibitin recruits Brg-1 and Brm to an E2F-responsive promoter. Jurkat and T47D cells were transfected with E2CAT and E2F1 or c-Fos–CAT reporters, with or without a prohibitin expression vector, as indicated. (A) CHIP assay using a pair of PCR primers against the CAT gene detected bands from the whole-cell lysate (‘Total’), indicating equivalent transfection efficiencies. The PCR assay using DNA recovered from the Brg-1 or Brm immunoprecipitates generated products only when prohibitin was co-transfected with E2CAT and E2F1, but not with c-Fos–CAT. PCR using DNA recovered from the p38 immunoprecipitate failed to detect any CAT gene. (B) CAT assay was performed using the same batches of cell lysates. Transfection of 2 µg of E2CAT along with 2 µg of E2F1 vector or 12 µg of c-Fos–CAT resulted in basal CAT activity (lanes 1 and 3). Co-transfection of a prohibitin expression vector (PH) repressed the transcription of E2CAT (lane 2), but not that of c-Fos–CAT (lane 4). Quantitation of CAT activity: the radioactivity recovered from the TLC plate was measured and the relative CAT activity was calculated based on ‘E2CAT + E2F1’ as ‘100’.
Fig. 4. Expression of Flag-tagged dnBrg-1 or dnBrm preverses prohibitin-mediated repression of E2F. (A) Cells carrying inducible dominant-negative Brg-1 (dnBrg-1) (B05-1) or inducible dominant-negative Brm (dnBrm) (H-16) were transfected with E2CAT and E2F1. Expression of the dominant-negative Brm or Brg-1 was confirmed by the immunoblot using a Flag antibody (lanes 4 and 8). Expression of transfected E2F (lanes 2–4, 6, 7 and 8) and prohibitin (lanes 3, 4, 7 and 8) was confirmed by immunoblot. Immunoblot of the same cell extracts with the p38 antibody confirmed equal loading. (B) CAT assay. Co-transfection of a prohibitin expression vector repressed the E2F-induced transcription (lanes 3 and 7) in the presence of tetracycline (absence of dnBrg-1 and dnBrm). Induction of dnBrg-1 and dnBrm expression by withdrawal of tetracycline from the medium reversed the prohibitin- mediated E2F repression (lanes 4 and 8). Quantitation of CAT activity: the radioactivity recovered from the TLC plate was measured and the relative activity was calculated based on ‘E2CAT + E2F1 + tetracycline’ as ‘100’.
Fig. 5. Rb is required for the prohibitin/Brg-1/Brm-mediated transcriptional repression. (A) Saos2 cells (lacking Rb) and C33A cells (Brg-1 and Brm deficient, and expressing a mutant Rb that cannot bind to Brg-1) were transfected with pSVECGCAT. The basal CAT activity was not affected by the co-transfection of GAL4–prohibitin, Brg-1 and Brm in Saos2 cells (lanes 1–4). The activity of pSVECGCAT was repressed when prohibitin was co-transfected with Brg-1 or Brm in C33A cells, while prohibitin, Brg-1 or Brm alone did not affect the activity (lanes 5–10). (B) Saos2 cell lysate was immunoprecipitated using either Brg-1, Brm or prohibitin antibodies followed by immunoblotting using prohibitin or Brg-1 and Brm antibodies. A c-Myc antibody was used as a negative control for the immunoprecipitation and a p38 antibody for the immunoblot analysis. (C) CHIP assay using the same Saos2 cell extracts shown in (A), which were transfected with pSVECGCAT and GAL4–prohibitin as indicated. The DNA recovered from immunoprecipitation by Brg-1, Brm, prohibitin or p38 (as negative control) antibodies was amplified by PCR, using primers against a region of the CAT gene. Co-transfection of the GAL4–prohibitin expression vector resulted in a PCR product using DNA recovered from the Brg-1, Brm or prohibitin immunoprecipitates, indicating that GAL4–prohibitin, Brg-1 and Brm are recruited to this promoter, which carries a GAL4-binding site. Direct PCR using the cell extracts without immunoprecipitation detected comparable amounts of the CAT gene from both extracts tested, demonstrating the equal transfection efficiency. PCR failed to detect the CAT gene in the p38 immunoprecipitates, indicating the specificity of the CHIP assay. (D) Saos2 cells were transfected with pSVECGCAT. Co-transfection of GAL4–prohibitin or Rb alone failed to repress the transcriptional activity of the reporter (lanes 1, 2 and 3). CAT activity was repressed by co-transfection of GAL4–prohibitin plus Rb expression vectors (lane 4), and this repression was reversed by co-transfection of dominant-negative Brg-1 (BJ5Brg1 K-R) or dominant-negative Brm (CGBrm NTP) (lanes 5–8).
Fig. 6. SV40T releases prohibitin-mediated E2F repression. (A) CAT assay. Jurkat and T47D cells were transfected with E2CAT and E2F1. The prohibitin- and Rb-mediated repression of E2F-dependent transcription (lanes 3 and 6) was released by co-transfection of an SV40T expression vector (lanes 4 and 7). Co-transfection of an E1A expression vector released Rb-mediated repression of E2F (lane 8), but had no effect on prohibitin-mediated repression (lane 5). Quantitation of CAT activity: the radioactivity of the TLC plate was measured and the relative activity was calculated based on ‘E2CAT + E2F1’ as ‘100’. (B) CHIP assay using extracts from Jurkat and T47D cells, which were transfected with the E2CAT, prohibitin and/or SV40T expression vectors as indicated. The DNA recovered from immunoprecipitation by Brg-1 or Brm antibodies was amplified by PCR, using primers against a region of the CAT gene. Transfection of a prohibitin expression vector resulted in a product in the PCR using DNA recovered from the Brg-1 and Brm immunoprecipitates, indicating that Brg-1 or Brm are recruited to this E2F-responsive promoter. Co-transfection of SV40T, however, generated no such PCR product, demonstrating that the recruitment of Brg-1 or Brm by prohibitin is blocked by SV40T. PCR using DNA recovered from the prohibitin immunoprecipitate detected the CAT gene in both prohibitin-transfected extracts, including extracts from cells that were co-transfected with SV40T, in which the prohibitin-mediated transcriptional repression was released. PCR using the DNA from the E2F immunoprecipitate showed equal association of E2F on the promoter of the transfected E2CAT. PCR failed to detect the CAT gene in the p38 immunoprecipitates, indicating the specificity of the CHIP assay.
Fig. 7. SV40T interacts with prohibitin in vivo. (A) Jurkat and HSF cells were transfected with an SV40T expression vector, and SV40T expression were confirmed by immunoblotting. Immunoblotting with a p38 antibody served as a loading control. (B and C) Immuno precipitation was performed using either an SV40T antibody or a prohibitin antibody, followed by immunoblot analysis using a prohibitin or SV40T antibody. A Myc antibody served as a control for the immunoprecipitations, and a p38 antibody as a control in the immunoblot experiments.
Fig. 8. SV40T blocks prohibitin-mediated recruitment of Brg-1 and Brm to native E2F-responsive promoters. (A) Immunoblot (IB) analysis showing the presence of the SV40T proteins in the Jurkat/SV40T cell line. As a loading control, equal levels of p38 protein were detected in both Jurkat and Jurkat/SV40T cells. (B) Jurkat or Jurkat/SV40T cell extracts were immunoprecipitated (IP) using Myc (as a negative control) or prohibitin (Proh) antibodies, followed by IB analysis using Brg-1, Brm or E2F1 antibodies. (C) Jurkat and Jurkat/SV40T cell extracts were immunoprecipitated using Myc (negative control), Brg-1, Brm or E2F1 antibodies, followed by IB analysis using the prohibitin antibody or a control antibody (p38). (D) CHIP assay using Jurkat or Jurkat/SV40T cell extracts as indicated. The DNA recovered from the immunoprecipitate by the indicated antibodies was amplified by PCR using primers against a region on one of three E2F-responsive promoters (TK, E2F1 or p107) and one non-E2F-responsive promoter (c-Fos) as a control. An amplified band was detected in the CHIP assay of the E2F-responsive promoters, but not of the c-Fos promoter, using the DNA recovered from Brg-1 or Brm immunoprecipitates from the Jurkat cell extract, indicating the specific association of Brg-1 or Brm with the E2F-responsive promoters. There was no PCR band detected using the DNA recovered from the Brg-1 or Brm immunoprecipitates from the Jurkat/SV40T cell extract for the same E2F-responsive promoters, demonstrating that the association of Brg-1 or Brm with the E2F-responsive promoters is blocked by the presence of SV40T (demonstrated in the CHIP assay using the SV40T antibody, Ab-2, as indicated). CHIP assay of the E2F promoters using prohibitin or E2F 1 antibodies resulted in an equal amount of PCR products using extracts from both Jurkat and Jurkat/SV40T cells, indicating that the associations between prohibitin or E2F1 proteins and the promoters were not affected by the presence of SV40T. CHIP assay using a non-related antibody against p38 generated no PCR band in any other reactions, demonstrating the specificity of the CHIP. PCR using DNA directly isolated from the cell extracts resulted in bands in all the lanes tested, serving as a positive control of the PCR (Total). RT–PCR assays demonstrated a relative increase in transcription of the E2F-responsive genes in the Jurkat/SV40T cells, compared with the parental Jurkat cells. Results from the control CHIP assay and RT–PCR of the non-E2F-responsive gene, c-Fos, confirmed the specificity of the assays.
Similar articles
- Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function.
Wang S, Nath N, Adlam M, Chellappan S. Wang S, et al. Oncogene. 1999 Jun 10;18(23):3501-10. doi: 10.1038/sj.onc.1202684. Oncogene. 1999. PMID: 10376528 - Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression.
Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Wang S, et al. Oncogene. 2002 Dec 5;21(55):8388-96. doi: 10.1038/sj.onc.1205944. Oncogene. 2002. PMID: 12466959 - Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals.
Wang S, Nath N, Fusaro G, Chellappan S. Wang S, et al. Mol Cell Biol. 1999 Nov;19(11):7447-60. doi: 10.1128/MCB.19.11.7447. Mol Cell Biol. 1999. PMID: 10523633 Free PMC article. - The mammalian SWI/SNF complex and the control of cell growth.
Muchardt C, Yaniv M. Muchardt C, et al. Semin Cell Dev Biol. 1999 Apr;10(2):189-95. doi: 10.1006/scdb.1999.0300. Semin Cell Dev Biol. 1999. PMID: 10441072 Review. - Molecular mechanisms of E2F-dependent activation and pRB-mediated repression.
Frolov MV, Dyson NJ. Frolov MV, et al. J Cell Sci. 2004 May 1;117(Pt 11):2173-81. doi: 10.1242/jcs.01227. J Cell Sci. 2004. PMID: 15126619 Review.
Cited by
- Suppression of mitochondrial ROS by prohibitin drives glioblastoma progression and therapeutic resistance.
Huang H, Zhang S, Li Y, Liu Z, Mi L, Cai Y, Wang X, Chen L, Ran H, Xiao D, Li F, Wu J, Li T, Han Q, Chen L, Pan X, Li H, Li T, He K, Li A, Zhang X, Zhou T, Xia Q, Man J. Huang H, et al. Nat Commun. 2021 Jun 17;12(1):3720. doi: 10.1038/s41467-021-24108-6. Nat Commun. 2021. PMID: 34140524 Free PMC article. - Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence.
Schleicher M, Shepherd BR, Suarez Y, Fernandez-Hernando C, Yu J, Pan Y, Acevedo LM, Shadel GS, Sessa WC. Schleicher M, et al. J Cell Biol. 2008 Jan 14;180(1):101-12. doi: 10.1083/jcb.200706072. J Cell Biol. 2008. PMID: 18195103 Free PMC article. - Interaction of HP1 and Brg1/Brm with the globular domain of histone H3 is required for HP1-mediated repression.
Lavigne M, Eskeland R, Azebi S, Saint-André V, Jang SM, Batsché E, Fan HY, Kingston RE, Imhof A, Muchardt C. Lavigne M, et al. PLoS Genet. 2009 Dec;5(12):e1000769. doi: 10.1371/journal.pgen.1000769. Epub 2009 Dec 11. PLoS Genet. 2009. PMID: 20011120 Free PMC article. - Proteomics Approach to Identify Serum Biomarkers Associated with Gastric Cancer in South Indian Tamils.
Madhesh JC, Narasiman M, Nagarajan V, Meenalosani S, Varshikaa S, Sivagnanam A, Jayaraman M. Madhesh JC, et al. Comb Chem High Throughput Screen. 2025;28(7):1229-1239. doi: 10.2174/0113862073302521240429112034. Comb Chem High Throughput Screen. 2025. PMID: 38752642 - The prohibitins (PHB) gene family in tomato: Bioinformatic identification and expression analysis under abiotic and phytohormone stresses.
Huang F, Ye X, Wang Z, Ding Y, Cai X, Yu L, Waseem M, Abbas F, Ashraf U, Chen X, Ke Y. Huang F, et al. GM Crops Food. 2021 Jan 2;12(1):535-550. doi: 10.1080/21645698.2021.1872333. Epub 2021 Mar 8. GM Crops Food. 2021. PMID: 33678114 Free PMC article.
References
- Adams P.D. and Kaelin,W.G.,Jr (1995) Transcriptional control by E2F. Semin. Cancer Biol., 6, 99–108. - PubMed
- Adams P.D. and Kaelin,W.G.,Jr (1996) The cellular effects of E2F overexpression. Curr. Top. Microbiol. Immunol., 208, 79–93. - PubMed
- Asamoto M. and Cohen,S.M. (1994) Prohibitin gene is overexpressed but not mutated in rat bladder carcinomas and cell lines. Cancer Lett., 83, 201–207. - PubMed
- Brehm A. and Kouzarides,T. (1999) Retinoblastoma protein meets chromatin. Trends Biochem. Sci., 24, 142–145. - PubMed
- Brehm A., Miska,E.A., McCance,D.J., Reid,J.L., Bannister,A.J. and Kouzarides,T. (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature, 391, 597–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous