Identification of a receptor-binding domain of Bordetella dermonecrotic toxin - PubMed (original) (raw)
Identification of a receptor-binding domain of Bordetella dermonecrotic toxin
Takeshi Matsuzawa et al. Infect Immun. 2002 Jul.
Abstract
Bordetella dermonecrotic toxin (DNT) stimulates the assembly of actin stress fibers and focal adhesions by deamidating or polyaminating Gln63 of the small GTPase Rho. DNT is an A-B toxin which is composed of an N-terminal receptor-binding (B) domain and a C-terminal enzymatically active (A) domain. In this study, to analyze the functional and structural organization of DNT, we prepared 10 clones of hybridoma producing anti-DNT monoclonal antibodies. One of these antibodies, 2B3, neutralized the effects of DNT on target cells when mixed with the toxin. When microinjected into cells, however, 2B3 did not inhibit the intoxication by DNT. Western blot analysis revealed that 2B3 recognized the N-terminal region of DNT. To delineate the DNT-binding domain, we examined a series of truncated DNT mutants for the ability to competitively inhibit the intoxication of cells by the full-length DNT and found that a fragment consisting of the N-terminal 54 amino acids (DNT(1-54)) was the smallest inhibitory fragment. The radioiodinated DNT(1-54) actually bound to target cells, which was inhibited by 2B3. These results suggest that the N-terminal 54 amino acids of DNT are responsible for the binding to target cells. DNT(1-54) bound to none of the DNT-resistant cells, implying the presence of a cell surface receptor specific to DNT-sensitive cells.
Figures
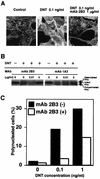
FIG. 1.
Neutralization of DNT by 2B3. (A) DNT (1 ng/ml) was incubated with (10 μg/ml) or without 2B3 at 37°C for 30 min and added to cultures of MC3T3-E1 cells at a final concentration of 0.1 ng/ml. The cells were incubated for 36 h and stained for actin cytoskeletons. (B) DNT (10 ng/ml) was treated with or without 0.1 and 50 μg of 2B3 or 1A3/ml, and the mixtures were diluted 10-fold and added to cultures of MC3T3-E1 cells. After incubation for 36 h, the cells were examined for modifications of Rho. Upper and lower mobility shifts of Rho represent deamidation and polyamination caused by DNT, respectively. (C) DNT at 1 or 10 ng/ml was incubated with or without 10 μg of 2B3/ml at 37°C for 30 min. A 10-fold dilution of the mixture was applied to MC3T3-E1 cells. After incubation for 36 h, the mono- and polynucleated cells were enumerated, and the percentage of polynucleated cells in at least 400 cells was calculated as described elsewhere (13). Three independent experiments were carried out, and representative data are shown.
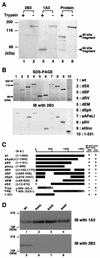
FIG. 2.
Localization of an epitope recognized by 2B3. (A) Trypsin-treated (lanes 1, 3, and 5) or nontreated (lanes 2, 4, and 6) DNT was sujected to Western blot analysis with 2B3 (lanes 1 and 2) or 1A3 (lanes 3 and 4). Total proteins electrotransferred on the membrane were stained with Coomassie brilliant blue R-250 (lanes 5 and 6).(B) The lysates of E. coli expressing DNT mutants were subjected to SDS-PAGE (upper panel) and Western blot analysis with 2B3 (lower panel). (C) A schematic representation of wild-type (wt) and deletion mutants of DNT. The results of the Western blot analyses are summarized on the right. The numbers in parentheses indicate the positions of amino acids covering the peptides or deletions (Δ). The C-terminal ends of the tryptic 60- and 90-kDa fragments were not identified. (D) His-DNTwt (lanes 1 and 5), His-DNT R44G (lanes 2 and 6), His-DNT R44S (lanes 3 and 7), and His-DNT R44K (lanes 4 and 8) were subjected to SDS-PAGE and immunoblotted (IB) with 1A3 (lanes 1 to 4) or 2B3 (lanes 5 to 8).
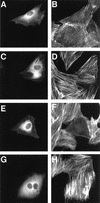
FIG. 3.
The DNT-induced formation of actin stress fibers in MC3T3-E1 cells microinjected with antibodies. The cells were microinjected with buffer alone (A to D), anti-DNT polyclonal antibody (E and F), or 2B3 (G and H). After incubation with (C to H) or without (A and B) 5 ng of DNT/ml for 20 h, the cells were stained for actin cytoskeletons (B, D, F, and H). Microinjected cells were distinguished by staining with Alexa 488-antibody against rabbit IgG microinjected along with the test samples (A, C, E, and G).
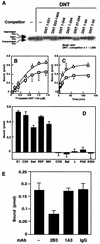
FIG. 4.
Definition of the binding domain of DNT. (A) Competitive inhibition of the Rho modifications caused by DNT with various deletion mutants. The mutant proteins were derived from pETDNT1-531-GST, pGEXGST-DNT523-1464, pETDNT1-344-GST, pETDNT1-244-GST, pETDNT47-244-GST, pETDNT1-94-GST, and pETDNT1-54-GST. MC3T3-E1 cells were incubated with DNT (5 ng/ml, ca. 31 pM) and each deletion mutant (31 nM) at 37°C for 8 h and examined for modifications of intracellular Rho. Numbers in the mutant namesindicate positions of the N-terminal and the C-terminal amino acids. (B) Dose-dependent binding of 125I-labeled DNT1-54 to the cells at 37°C (○) or 4°C (□). (C) Time course of the binding of 125I-labeled DNT1-54 to MC3T3-E1 cells at 37°C (○) or 4°C (□). (D) Binding of 125I-labeled DNT1-54 to MC3T3-E1 (E1), C3H10T1/2 (C3H), Swiss 3T3 (Swi), rat embryo fibroblasts (REF), NIH 3T3 (NIH), COS7 (COS), BALB 3T3 (Bal), L929 (L), PAE, and K562. 125I-labeled DNT1-54 (1.2 μM) was incubated with each cell line at 37°C for 1 h. (E) Inhibition of the binding of DNT1-54 to the cells by 2B3. 125I-DNT1-54 at 0.21 μM was preincubated with or without 850 μg of 2B3, 1A3, or normal IgG/ml at 37°C for 30 min and then incubated with MC3T3-E1 cells at a final concentration of 0.15 μM for 1 h. (B to E) Three independent experiments were performed, and representative data (means ± the standard deviations of three samples) are shown.
Similar articles
- Immunological and protective effects of Bordetella bronchiseptica subunit vaccines based on the recombinant N-terminal domain of dermonecrotic toxin.
Wang C, Liu L, Zhang Z, Yan Z, Yu C, Shao M, Jiang X, Chi S, Wei K, Zhu R. Wang C, et al. Int Immunopharmacol. 2015 Oct;28(2):952-9. doi: 10.1016/j.intimp.2015.08.018. Epub 2015 Sep 1. Int Immunopharmacol. 2015. PMID: 26337750 - Identification of functional domains of Bordetella dermonecrotizing toxin.
Kashimoto T, Katahira J, Cornejo WR, Masuda M, Fukuoh A, Matsuzawa T, Ohnishi T, Horiguchi Y. Kashimoto T, et al. Infect Immun. 1999 Aug;67(8):3727-32. doi: 10.1128/IAI.67.8.3727-3732.1999. Infect Immun. 1999. PMID: 10417130 Free PMC article. - [Expression and characterization of the dermonecrotic toxin gene of Bordetella bronchiseptica].
Xue Y, Zhao Z, Pei J, Wang C, Ding K, Cheng X. Xue Y, et al. Sheng Wu Gong Cheng Xue Bao. 2011 Dec;27(12):1722-8. Sheng Wu Gong Cheng Xue Bao. 2011. PMID: 22506412 Chinese. - Swine atrophic rhinitis caused by pasteurella multocida toxin and bordetella dermonecrotic toxin.
Horiguchi Y. Horiguchi Y. Curr Top Microbiol Immunol. 2012;361:113-29. doi: 10.1007/82_2012_206. Curr Top Microbiol Immunol. 2012. PMID: 22411430 Review.
Cited by
- Polymorphisms influencing expression of dermonecrotic toxin in Bordetella bronchiseptica.
Okada K, Abe H, Ike F, Ogura Y, Hayashi T, Fukui-Miyazaki A, Nakamura K, Shinzawa N, Horiguchi Y. Okada K, et al. PLoS One. 2015 Feb 2;10(2):e0116604. doi: 10.1371/journal.pone.0116604. eCollection 2015. PLoS One. 2015. PMID: 25642712 Free PMC article. - Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice.
Dufies O, Doye A, Courjon J, Torre C, Michel G, Loubatier C, Jacquel A, Chaintreuil P, Majoor A, Guinamard RR, Gallerand A, Saavedra PHV, Verhoeyen E, Rey A, Marchetti S, Ruimy R, Czerucka D, Lamkanfi M, Py BF, Munro P, Visvikis O, Boyer L. Dufies O, et al. Nat Microbiol. 2021 Mar;6(3):401-412. doi: 10.1038/s41564-020-00832-5. Epub 2021 Jan 11. Nat Microbiol. 2021. PMID: 33432150 Free PMC article. - Cellular and molecular action of the mitogenic protein-deamidating toxin from Pasteurella multocida.
Wilson BA, Ho M. Wilson BA, et al. FEBS J. 2011 Dec;278(23):4616-32. doi: 10.1111/j.1742-4658.2011.08158.x. Epub 2011 May 31. FEBS J. 2011. PMID: 21569202 Free PMC article. Review. - Modulation of connexin signaling by bacterial pathogens and their toxins.
Ceelen L, Haesebrouck F, Vanhaecke T, Rogiers V, Vinken M. Ceelen L, et al. Cell Mol Life Sci. 2011 Sep;68(18):3047-64. doi: 10.1007/s00018-011-0737-z. Epub 2011 Jun 9. Cell Mol Life Sci. 2011. PMID: 21656255 Free PMC article. Review. - Bordetella Pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance.
Dorji D, Mooi F, Yantorno O, Deora R, Graham RM, Mukkur TK. Dorji D, et al. Med Microbiol Immunol. 2018 Feb;207(1):3-26. doi: 10.1007/s00430-017-0524-z. Epub 2017 Nov 21. Med Microbiol Immunol. 2018. PMID: 29164393 Review.
References
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
- Bruckner, I. E., and D. G. Evans. 1939. The toxin of B. parapertussis and the relationship of this organism to H. pertussis and B. bronchiseptica. J. Pathol. Bacteriol. 48:67-78.
- Evans, D. G. 1940. The production of pertussis antitoxin in rabbits and the neutralization of pertussis, parapertussis, and bronchiseptica toxins. J. Pathol. Bacteriol. 51:49-58.
- Hanada, M., K. Shimoda, S. Tomita, Y. Nakase, and Y. Nishiyama. 1979. Production of lesions similar to naturally occurring swine atrophic rhinitis by cell-free sonicated extract of Bordetella bronchiseptica. Jpn. J. Vet. Sci. 41:1-8. - PubMed
- Horiguchi, Y., N. Inoue, M. Masuda, T. Kashimoto, J. Katahira, N. Sugimoto, and M. Matsuda. 1997. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc. Natl. Acad. Sci. USA 94:11623-11626. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases