Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation - PubMed (original) (raw)
Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation
Haroula Angelakopoulos et al. Infect Immun. 2002 Jul.
Abstract
Listeria monocytogenes is an intracellular bacterial pathogen which causes bacteremia and has a tropism for the central nervous system and a propensity to cause maternofetal infection. L. monocytogenes has been shown to be an effective prophylactic and a therapeutic vaccine vector for viral and tumor antigens in animal models. L. monocytogenes mutants lacking the ActA protein, which is essential for intracellular movement, are attenuated but retain immunogenicity in mice. Given the pathogenic potential of L. monocytogenes, we created an attenuated mutant strain bearing double deletions in the actA and plcB virulence genes for an initial clinical safety study of a prototype L. monocytogenes vector in adults. Twenty healthy volunteers received single escalating oral doses (10(6) to 10(9) CFU, 4 volunteers per dose cohort) of this attenuated L. monocytogenes, designated LH1169. Volunteers were monitored in the hospital for 14 days with frequent clinical checks and daily blood and stool cultures, and they were monitored for six additional weeks as outpatients. There were no positive blood cultures and no fevers attributable to the investigational inoculation. Most volunteers shed vaccine bacteria for 4 days or less, without diarrhea. One volunteer had a late positive stool culture during outpatient follow-up. Three volunteers had abnormal liver function test results temporally associated with inoculation; one could be reasonably attributed to another cause. In the highest-dose cohort, humoral, mucosal, and cellular immune responses to the investigational organism were detected in individual volunteers. Attenuated L. monocytogenes can be studied in adult volunteers without serious long-term health sequelae.
Figures
FIG. 1.
Genotype of _actA/plcB_-deleted L. monocytogenes LH1169. In-frame-defined deletions (denoted by white segments) were created as shown for the actA and plcB genes. The photograph shows a 1% agarose gel of PCR-amplified loci from wild-type L. monocytogenes 10403S (WT), a clinical isolate (C), the vaccine strain LH1169 (V), and the last fecal isolate obtained from volunteer 20 (#20). Lanes designated V and #20 show the expected truncated versions of amplicons spanning the actA locus (1.3 kb versus 2.3 kb for WT) and the plcB locus (1.1 kb versus 1.8 kb for WT). Std, 1-kb molecular size standard.
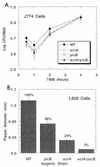
FIG. 2.
Survival of isogenic L. monocytogenes mutants within macrophage-like J774 cells and plaquing within fibroblast-like L929 cells. (A) J774 cells were infected for 30 min with wild type (WT), single mutants, or double mutants at a multiplicity of infection of 10:1 and were subsequently treated with gentamicin (20 μg/ml) to eliminate extracellular bacteria. Cells were lysed and bacteria were serially diluted and spread plated for CFU determinations on brain heart infusion agar. Data are presented as means of 3 wells ± standard errors. (B) L929 cells were similarly infected and overlaid with soft agar containing gentamicin (20 μg/ml). After 4 days of culture cells were overlaid with agar containing vital red dye, which stains live cells, for another day. Unstained, devitalized cell plaques are measured in millimeters. Mean plaque sizes ± standard errors of 10 plaques/strain are reported. Percentages reflect the percent of wild-type plaque size for mutants and show that the double mutant is highly limited in cell-to-cell spread, more so than either single mutant.
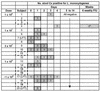
FIG. 3.
Fecal cultures by volunteer and day. The numbers represent the total number of stool samples positive for L. monocytogenes on that day. Shading denotes days on which at least one sample grew L. monocytogenes. A bolded outline represents days on which at least one culture was positive by direct plating of feces, an indication of larger organism burden. On days without bolded outlines, enrichment broth incubation was needed to detect the organism. Five volunteers (dashed) never had L. monocytogenes detected in any fecal sample. Volunteers with abnormal transaminases are noted (LFT). a, Only one volunteer (number 4) had a late positive culture at the sixth outpatient follow-up visit, after multiple previous samples had been negative (see the text for details).
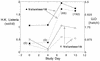
FIG. 4.
Time course of serum alanine aminotransferase (SGPT) and CPK in three volunteers with abnormal findings. Volunteers were inoculated on day 0 and had serum studies performed on the days indicated. (A) Volunteer 17, who received approximately 109 CFU, had a reasonable alternative explanation for aminotransferase elevations (vigorous exercise) and a concurrent elevation in CPK, a muscle enzyme. This individual was asymptomatic, and abnormalities resolved promptly with cessation of exercise. (B) Volunteer 7 (107 CFU) and volunteer 20 (109 CFU) were not exercising during the inpatient stay and had CPKs below 100. Volunteer 20 was minimally symptomatic, with intermittent right-upper-quadrant discomfort (horizontal bar); volunteer 7 was asymptomatic. Alkaline phosphatase and bilirubin determinations were within normal ranges for all subjects. Aspartate aminotransferases (ALT/SGOT) paralleled changes in SGPT in magnitude and time course and are not shown.
FIG. 5.
Measurement of soluble IgG directed against recombinant LLO or heat-killed L. monocytogenes (H.K. Listeria) by ELISA in tissue culture supernatants. PBMC were isolated from volunteers on the days designated and were cultured at 107 cells/ml for 48 h. Tissue culture media were aspirated, centrifuged free of cells, and applied to ELISA plates coated with vaccine antigens; peroxidase-labeled goat anti-human antibody was used to develop wells. Data shown are OD450 values (mean value of 2 wells). Control wells without antigens had OD values of ≤0.05. None of the 18 other volunteers had increases over day 0 values in this assay. Numbers in parentheses are mean IgG-bearing cells by traditional ELISPOT assay in the one volunteer who had positive results in those assays. We have arbitrarily designated a threefold or greater increase over baseline as a positive result in prior studies of this type; although these results are encouraging, they do not consistently meet that criterion, with the exception of volunteer 18's response to recombinant LLO.
FIG. 6.
Serum IgG by ELISA directed against L. monocytogenes 10403S. Sera on study days 0, 4, 7, 10, and 14 and weekly thereafter for 6 weeks were serially diluted twofold across ELISA plates coated with heat-killed wild-type L. monocytogenes. Total IgG was detected by addition of phosphatase-labeled goat anti-human IgG and _para_-nitrophenylphosphate substrate. Endpoint titers were defined as the final dilution at which OD405 was ≥0.15. Preimmune (Pre) and peak values of reciprocal titers are plotted for inoculated subjects. Control subjects were healthy, uninoculated individuals who agreed to provide serum samples on two separate occasions 14 to 18 days apart. Geometric mean titers are represented by horizontal bars. No subject in either group had a fourfold or greater difference in titer, and the differences between groups (day 0 versus day 14, preimmune to peak values, and day 0 versus preimmune) were not statistically significant by the Mann-Whitney test. The datum for one patient with bacteremic clinical listeriosis who had a prominent response in this assay is also plotted (Pos. Control).
Similar articles
- Attenuated Listeria monocytogenes vaccine vectors expressing influenza A nucleoprotein: preclinical evaluation and oral inoculation of volunteers.
Johnson PV, Blair BM, Zeller S, Kotton CN, Hohmann EL. Johnson PV, et al. Microbiol Immunol. 2011 May;55(5):304-17. doi: 10.1111/j.1348-0421.2011.00322.x. Microbiol Immunol. 2011. PMID: 21338384 Free PMC article. Clinical Trial. - Pathogenicity and immunogenicity of a mutant strain of Listeria monocytogenes in the chicken infection model.
Yin Y, Tian D, Jiao H, Zhang C, Pan Z, Zhang X, Wang X, Jiao X. Yin Y, et al. Clin Vaccine Immunol. 2011 Mar;18(3):500-5. doi: 10.1128/CVI.00445-10. Epub 2011 Jan 12. Clin Vaccine Immunol. 2011. PMID: 21228136 Free PMC article. - [Construction and characterization of a mutant strain of Listeria monocytogenes with a deletion of actA and plcB].
Yin Y, Zhu G, Geng S, Hu M, Jiao X. Yin Y, et al. Wei Sheng Wu Xue Bao. 2008 Mar;48(3):299-303. Wei Sheng Wu Xue Bao. 2008. PMID: 18479054 Chinese. - Molecular aspects of Listeria monocytogenes infection.
Krawczyk-Balska A, Bielecki J. Krawczyk-Balska A, et al. Pol J Microbiol. 2004;53 Suppl:17-22. Pol J Microbiol. 2004. PMID: 15787192 Review. - ActA of Listeria monocytogenes and Its Manifold Activities as an Important Listerial Virulence Factor.
Pillich H, Puri M, Chakraborty T. Pillich H, et al. Curr Top Microbiol Immunol. 2017;399:113-132. doi: 10.1007/82_2016_30. Curr Top Microbiol Immunol. 2017. PMID: 27726006 Review.
Cited by
- Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies.
Kim VM, Blair AB, Lauer P, Foley K, Che X, Soares K, Xia T, Muth ST, Kleponis J, Armstrong TD, Wolfgang CL, Jaffee EM, Brockstedt D, Zheng L. Kim VM, et al. J Immunother Cancer. 2019 May 22;7(1):132. doi: 10.1186/s40425-019-0601-5. J Immunother Cancer. 2019. PMID: 31113479 Free PMC article. - YopE specific CD8+ T cells provide protection against systemic and mucosal Yersinia pseudotuberculosis infection.
González-Juarbe N, Shen H, Bergman MA, Orihuela CJ, Dube PH. González-Juarbe N, et al. PLoS One. 2017 Feb 16;12(2):e0172314. doi: 10.1371/journal.pone.0172314. eCollection 2017. PLoS One. 2017. PMID: 28207901 Free PMC article. - GNP-GAPDH1-22 nanovaccines prevent neonatal listeriosis by blocking microglial apoptosis and bacterial dissemination.
Calderon-Gonzalez R, Frande-Cabanes E, Teran-Navarro H, Marimon JM, Freire J, Salcines-Cuevas D, Carmen Fariñas M, Onzalez-Rico C, Marradi M, Garcia I, Alkorta-Gurrutxaga M, San Nicolas-Gomez A, Castañeda-Sampedro A, Yañez-Diaz S, Penades S, Punzon C, Gomez-Roman J, Rivera F, Fresno M, Alvarez-Dominguez C. Calderon-Gonzalez R, et al. Oncotarget. 2017 Jul 20;8(33):53916-53934. doi: 10.18632/oncotarget.19405. eCollection 2017 Aug 15. Oncotarget. 2017. PMID: 28903312 Free PMC article. - A Promising Listeria-Vectored Vaccine Induces Th1-Type Immune Responses and Confers Protection Against Tuberculosis.
Yin Y, Lian K, Zhao D, Tao C, Chen X, Tan W, Wang X, Xu Z, Hu M, Rao Y, Zhou X, Pan Z, Zhang X, Jiao X. Yin Y, et al. Front Cell Infect Microbiol. 2017 Sep 28;7:407. doi: 10.3389/fcimb.2017.00407. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29034213 Free PMC article. - CD8(+) T cells specific to a single Yersinia pseudotuberculosis epitope restrict bacterial replication in the liver but fail to provide sterilizing immunity.
Shen H, Gonzalez-Juarbe N, Blanchette K, Crimmins G, Bergman MA, Isberg RR, Orihuela CJ, Dube PH. Shen H, et al. Infect Genet Evol. 2016 Sep;43:289-96. doi: 10.1016/j.meegid.2016.06.008. Epub 2016 Jun 4. Infect Genet Evol. 2016. PMID: 27268148 Free PMC article.
References
- Aureli, P., G. C. Fiorucci, D. Caroli, G. Marchiaro, O. Novara, L. Leone, and S. Salmaso. 2000. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N. Engl. J. Med. 342:1236-1241. - PubMed
- Boury, N. M., and C. J. Czuprynski. 1995. Listeria monocytogenes infection increases neutrophil adhesion and damage to a murine hepatocyte cell line in vitro. Immunol. Lett. 46:111-116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI045137/AI/NIAID NIH HHS/United States
- R01CA84008/CA/NCI NIH HHS/United States
- R01 CA084008/CA/NCI NIH HHS/United States
- R01AI45137/AI/NIAID NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- M01RR01066-21/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous