Vesicle pool partitioning influences presynaptic diversity and weighting in rat hippocampal synapses - PubMed (original) (raw)
Vesicle pool partitioning influences presynaptic diversity and weighting in rat hippocampal synapses
Jack Waters et al. J Physiol. 2002.
Abstract
Hippocampal synapses display a range of release probabilities. This is partially the result of scaling of release probability with the total number of releasable vesicles at each synapse. We have compared synaptic release and vesicle pool sizes across a large number of hippocampal synapses using FM 1-43 and confocal fluorescence microscopy. We found that the relationship between the number of recycling vesicles at a synapse and its release probability is dependent on firing frequency. During firing at 10 Hz, the release probability of each synapse is closely related to the number of recycling vesicles that it contains. In contrast, during firing at 1 Hz, different synapses turn over their recycling vesicle pools at different rates leading to an indirect relationship between recycling vesicle pool size and release probability. Hence two synapses may release vesicles at markedly different rates during low frequency firing, even if they contain similar numbers of vesicles. Both further kinetic analyses and manipulation of the number of vesicles in the readily releasable pool using phorbol ester treatment suggested that this imprecise scaling observed during firing at 1 Hz resulted from synapse-to-synapse differences in the proportion of recycling vesicles partitioned into the readily releasable pool. Hence differential partitioning between vesicle pools affects presynaptic weighting in a frequency-dependent manner. Since hippocampal single unit firing rates shift between 1 Hz and 10 Hz regimes with behavioural state, differential partitioning may be a mechanism for encoding information in hippocampal circuits.
Figures
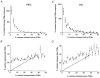
Figure 2. Pronounced kinetic heterogeneity at 1 Hz, but not 10 Hz
A, fluorescence intensities of 40 individual fluorescent puncta followed through time. Traces begin in the stained condition. Staining consisted of 900 stimuli at 10 Hz, followed by an additional minute in FM 1-43 then a 10 min wash period. During stimulation at 10 Hz, each punctum loses fluorescence through time. Note that all puncta are fully destained by 900 stimuli at 10 Hz. Data were corrected for non-specific staining by subtracting the fluorescence intensity after 1200 stimuli. B, the same data following normalization to the fully stained condition. C, similar data derived using the same loading protocol, but using a 1 Hz destaining stimulus train. Note that some synapses destained only very slowly, if at all, necessitating the use of a 10 Hz stimulus train (arrow) to complete destaining. Data from the fully destained condition are visible on the right.
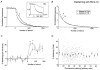
Figure 5. Effect of PDBu at 10 Hz
A, 10 Hz destaining kinetics measured with FM 1-43 before and after PDBu treatment. Data points represent mean (±
s.e.m.
) measurements from 141 puncta. B, same data represented as percentage destaining between each image, as in Fig. 4_C_. The effect of PDBu was significant after 30 and 60 destaining stimuli (P < 0.01, Wilcoxon's signed rank test) where destaining was increased by 110 % and 25 % respectively. C, 10 Hz destaining kinetics measured with FM 2-10 before and after PDBu treatment. Data points represent mean (±
s.e.m.
) measurements from 138 puncta. D, percentage destaining between each image for destaining curves derived using FM 2-10. The effect of PDBu was significant after 30 and 60 destaining stimuli (P < 0.01, Wilcoxon's signed rank test). The degree of potentiation was also similar to that with FM 1-43, being 115 % and 50 % after 30 and 60 stimuli, respectively.
Figure 1. Example of sequential FM 1-43 staining and destaining
A, Nomarski image of a mixed neuronal-glial culture after 13 days in vitro. B, the _s_ame field after staining with FM 2-10. The staining protocol consisted of 100 stimuli at 10 Hz then removal of extracellular dye 30 s after cessation of the stimulus train. C, fluorescence image following destaining with a 900 stimulus train at 10 Hz. Remaining fluorescence represents non-specific staining, all vesicular staining having been released. D, composite image comparing staining during subsequent trials. Data were corrected for non-specific staining. Red represents the first round of staining (image C subtracted from image B) and green the second round (images E - F), yellow denotes regions where red and green overlap. Note that almost all puncta appear in the same position in the two images indicating that these synapses are positionally stable. E and F, fluorescence images from a second round of staining and destaining using an identical protocol to that for images B and C. Comparison of E with C reveals an increase in non-specific staining. Note also that fluorescence intensities are similar following 1st and 2nd rounds of staining. Scale bar represents 10 μm.
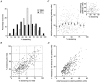
Figure 3. Properties of 1 Hz heterogeneity
A, frequency histogram comparing the extent of destaining following 150 stimuli at 1 Hz and 10 Hz. Frequency is displayed as the percentage, rather than absolute number, of puncta in each bin to allow direct comparison of 1 Hz and 10 Hz data, despite different numbers of observations. Data represent 616 puncta at 1 Hz and 375 puncta at 10 Hz. B, scatter plot illustrating the 1 Hz fractional destaining rates of 333 puncta during two subsequent trials. Each point represents the destaining percentages of a single fluorescent punctum after 150 stimuli at 1 Hz in each trial. The data were best fitted with a regression line of slope 1.009 passing through the origin. C, scatter plot comparing 1 Hz fractional destaining rates with initial fluorescence intensity in the stained condition. The staining protocol consisted of 100 stimuli at 10 Hz. Fractional destaining rates are represented as percentage destaining after 150 stimuli at 1 Hz. Small points represent the values of 333 individual puncta. Large circular symbols represent the mean (±
s.e.m.
) values for the same data binned according to their rates of destaining (bins each represent 10 percentage units). Inset, scatter plot comparing 1 Hz fractional destaining rates with fluorescence intensity in the stained condition for a single experiment (108 puncta). D, plots showing the absolute amount of fluorescence released in response to 150 stimuli at 1 Hz as a function of initial fluorescence staining intensity. Each of the 333 data points represents data from one synapse.
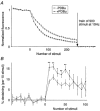
Figure 4. Direct comparison of 1 Hz and 10 Hz destaining curves
A, comparison of the fractional destaining rates at 10 Hz of two groups of puncta stained with FM 2-10: those with rapid and those with slow fractional rates at 1 Hz (filled and open circles, respectively). Data points represent the mean (±
s.e.m.
) of 50 puncta (of a total population of 214). Inset, mean destaining curves at 1 Hz for the same two groups. The asterisk denotes the data point after 150 stimuli (see below). B, the same 10 Hz curves (presented in A) normalized to the fluorescence intensity after 150 destaining stimuli. Note that the curves converge after the first few images. To analyse this phenomenon in more detail the percentage destaining occurring between each image pair was calculated for each punctum. For instance, the percentage destaining occurring between images labelled A and B was calculated using the following formula: % destaining = 100 ×[(intensity in A) - (intensity in B)]/(intensity in A). These data are presented in C. C, mean (±
s.e.m.
) destaining per image pair. Values were calculated separately for each punctum then pooled as in A. Asterisks denote a significant difference between destaining rates (P < 0.01, Mann-Whitney rank sum test). D, scatter plot comparing percentage destaining by 30 stimuli at 10 Hz and total pool size. Data were derived from experiments using FM 1-43. Small points represent 476 individual puncta and large circular symbols represent mean (±
s.e.m.
) of the same data grouped into 3-unit bins.
Figure 6. Effect of PDBu at 1 Hz
A, 1 Hz destaining curves generated with FM 1-43 before and after PDBu treatment. Data points represent mean (±
s.e.m.
) measurements from 78 puncta. B, same data represented as percentage destaining between each image, as in Fig. 4_C_. The effect of PDBu was significant after 10, 20, 30, 40, 50 and 60 destaining stimuli (**P < 0.01, *P < 0.05, Wilcoxon's signed rank test) where destaining was increased by 78 %, 46 %, 54 %, 74 %, 78 % and 74 %, respectively.
Figure 7. Effect of PDBu depends on initial synaptic properties
A, plot to show the relationship between the effect of PDBu and the proportion of recycling vesicles in the readily releasable pool before PDBu treatment. Release was induced by 30 stimuli at 10 Hz. Data from 774 puncta were binned according to the percentage released before PDBu treatment (2-unit bins) and are plotted as means (±
s.e.m.
). B, same data presented to compare release before and after PDBu treatment. The line indicates the relationship expected if PDBu treatment were excluded. C and D, similar data to those presented in A and B, but using a 1 Hz stimulus (30 stimuli). Data represent a total of 417 puncta.
Similar articles
- Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms.
Waters J, Smith SJ. Waters J, et al. J Neurosci. 2000 Nov 1;20(21):7863-70. doi: 10.1523/JNEUROSCI.20-21-07863.2000. J Neurosci. 2000. PMID: 11050105 Free PMC article. - Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus.
Dobrunz LE. Dobrunz LE. Int J Dev Neurosci. 2002 Jun-Aug;20(3-5):225-36. doi: 10.1016/s0736-5748(02)00015-1. Int J Dev Neurosci. 2002. PMID: 12175858 - Vesicle pool mobilization during action potential firing at hippocampal synapses.
Ryan TA, Smith SJ. Ryan TA, et al. Neuron. 1995 May;14(5):983-9. doi: 10.1016/0896-6273(95)90336-4. Neuron. 1995. PMID: 7748565 - [Research progress of synaptic vesicle recycling].
Li YF, Zhang XX, Duan SM. Li YF, et al. Sheng Li Xue Bao. 2015 Dec 25;67(6):545-60. Sheng Li Xue Bao. 2015. PMID: 26701630 Review. Chinese. - [Presynaptic mechanisms of learning and memory].
Yawo H, Ishizuka T. Yawo H, et al. Brain Nerve. 2008 Jul;60(7):725-36. Brain Nerve. 2008. PMID: 18646612 Review. Japanese.
Cited by
- A preferentially segregated recycling vesicle pool of limited size supports neurotransmission in native central synapses.
Marra V, Burden JJ, Thorpe JR, Smith IT, Smith SL, Häusser M, Branco T, Staras K. Marra V, et al. Neuron. 2012 Nov 8;76(3):579-89. doi: 10.1016/j.neuron.2012.08.042. Neuron. 2012. PMID: 23141069 Free PMC article. - Optical tracking of phenotypically diverse individual synapses on solitary tract nucleus neurons.
Jin YH, Cahill EA, Fernandes LG, Wang X, Chen W, Smith SM, Andresen MC. Jin YH, et al. Brain Res. 2010 Feb 2;1312:54-66. doi: 10.1016/j.brainres.2009.11.042. Epub 2009 Nov 26. Brain Res. 2010. PMID: 19944080 Free PMC article. - Functional analysis of calcium channel-mediated exocytosis in synaptic terminals by FM imaging technique.
Gu F, Fu L, Ma YJ. Gu F, et al. Neurosci Bull. 2009 Aug;25(4):216-20. doi: 10.1007/s12264-009-0507-1. Neurosci Bull. 2009. PMID: 19633704 Free PMC article. - Dynamic Partitioning of Synaptic Vesicle Pools by the SNARE-Binding Protein Tomosyn.
Cazares VA, Njus MM, Manly A, Saldate JJ, Subramani A, Ben-Simon Y, Sutton MA, Ashery U, Stuenkel EL. Cazares VA, et al. J Neurosci. 2016 Nov 2;36(44):11208-11222. doi: 10.1523/JNEUROSCI.1297-16.2016. J Neurosci. 2016. PMID: 27807164 Free PMC article. - Use dependence of presynaptic tenacity.
Fisher-Lavie A, Zeidan A, Stern M, Garner CC, Ziv NE. Fisher-Lavie A, et al. J Neurosci. 2011 Nov 16;31(46):16770-80. doi: 10.1523/JNEUROSCI.3384-11.2011. J Neurosci. 2011. PMID: 22090503 Free PMC article.
References
- Betz W, Mao F, Smith C. Imaging exocytosis and endocytosis. Current Opinion in Neurobiology. 1996;6:365–371. - PubMed
- Czurkó A, Hirase H, Csicsvari J, Buzsáki G. Sustained activity of hippocampal pyramidal cells by ‘space clamping’ a running wheel. European Journal of Neuroscience. 1999;11:344–352. - PubMed
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. - PubMed
- Dubé GR, Marshall KC. Activity-dependent activation of presynaptic metabotropic glutamate receptors in locus coeruleus. Journal of Neurophysiology. 2000;83:1141–1149. - PubMed
- Goda Y, Südhof TC. Calcium regulation of neurotransmitter release: reliably unreliable? Current Opinion in Cell Biology. 1997;9:513–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources