Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori - PubMed (original) (raw)
Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori
Gary Sisson et al. Antimicrob Agents Chemother. 2002 Jul.
Abstract
Nitazoxanide (NTZ) is a redox-active nitrothiazolyl-salicylamide prodrug that kills Helicobacter pylori and also many anaerobic bacterial, protozoan, and helminthic species. Here we describe development and use of a spectrophotometric assay, based on nitroreduction of NTZ at 412 nm, to identify H. pylori enzymes responsible for its activation and mode of action. Three enzymes that reduce NTZ were identified: two related NADPH nitroreductases, which also mediate susceptibility to metronidazole (MTZ) (RdxA and FrxA), and pyruvate oxidoreductase (POR). Recombinant His-tagged RdxA, FrxA, and POR, overexpressed in nitroreductase-deficient Escherichia coli, each rapidly reduced NTZ, whereas only FrxA and to a lesser extent POR reduced nitrofuran substrates (furazolidone, nitrofurantoin, and nitrofurazone). POR exhibited no MTZ reductase activity either in extracts of H. pylori or following overexpression in E. coli; RdxA exhibited no nitrofuran reductase activity, and FrxA exhibited no MTZ reductase activity. Analysis of mutation to rifampin resistance (Rif(r)) indicated that NTZ was not mutagenic and that nitrofurans were only weakly mutagenic. Alkaline gel DNA electrophoresis indicated that none of these prodrugs caused DNA breakage. In contrast, MTZ caused DNA damage and was strongly mutagenic. We conclude that POR, an essential enzyme, is responsible for most or all of the bactericidal effects of NTZ against H. pylori. While loss-of-function mutations in rdxA and frxA produce a Mtz(r) phenotype, they do not contribute much to the innate susceptibility of H. pylori to NTZ or nitrofurans.
Figures
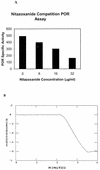
FIG. 1.
POR assay and competition with benzyl viologen. (A) Competitive inhibition of POR activity (benzyl viologen reduction) in H. pylori extracts as a function of NTZ concentration was monitored spectrophotometrically at 546 nm. The specific activity at each concentration of nitazoxanide was recorded. (B) POR was assayed in cell extracts of H. pylori by monitoring the pyruvate-dependent reduction of nitazoxanide at 412 nm (A) as described in the text.
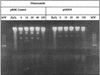
FIG. 2.
Lack of NTZ- or nitrofuran-induced DNA fragmentation in H. pylori. The MTZs strain H. pylori (Hp) 26695 was challenged with various concentrations of NTZ or nitrofurazone for 30 min as described in the text. The bacteria were suspended and lysed in agarose plugs, and agarose gels were run under alkaline conditions to display the extent of DNA fragmentation of denatured genomic DNA. Bacteria were treated with hydrogen peroxide (20 mM) for 15 min (positive controls). wt, wild type.
FIG. 3.
Lack of drug-induced DNA fragmentation of E. coli strain CC104 carrying pBSK. The bacteria were grown in the presence of the nitrofuran drugs as described in the text. The preparation of agarose plugs is as described in Fig. 2. Hydrogen peroxide was added at a 20 mM concentration as a positive control. The distinct bands noted in each of the lanes are pBSK plasmid DNA.
FIG. 4.
Lack of nitazoxanide-induced DNA fragmentation of E. coli strains carrying rdxA of H. pylori. E. coli strain CC104 containing either pBSK (control) or pGS950 (rdxA+) was grown in the presence of NTZ, and bacteria were suspended and lysed in agarose plugs and electrophoresed as described for Fig. 2 and detailed in the text. Hydrogen peroxide was added at a 20 mM concentration as a positive control. The distinct bands noted in the various lanes are plasmid DNA.
Similar articles
- Characterization of the NAD(P)H oxidase and metronidazole reductase activities of the RdxA nitroreductase of Helicobacter pylori.
Olekhnovich IN, Goodwin A, Hoffman PS. Olekhnovich IN, et al. FEBS J. 2009 Jun;276(12):3354-64. doi: 10.1111/j.1742-4658.2009.07060.x. Epub 2009 May 7. FEBS J. 2009. PMID: 19438716 Free PMC article. - Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori.
Jeong JY, Mukhopadhyay AK, Dailidiene D, Wang Y, Velapatiño B, Gilman RH, Parkinson AJ, Nair GB, Wong BC, Lam SK, Mistry R, Segal I, Yuan Y, Gao H, Alarcon T, Brea ML, Ito Y, Kersulyte D, Lee HK, Gong Y, Goodwin A, Hoffman PS, Berg DE. Jeong JY, et al. J Bacteriol. 2000 Sep;182(18):5082-90. doi: 10.1128/JB.182.18.5082-5090.2000. J Bacteriol. 2000. PMID: 10960091 Free PMC article. - Characterization of the genes rdxA and frxA involved in metronidazole resistance in Helicobacter pylori.
Marais A, Bilardi C, Cantet F, Mendz GL, Mégraud F. Marais A, et al. Res Microbiol. 2003 Mar;154(2):137-44. doi: 10.1016/S0923-2508(03)00030-5. Res Microbiol. 2003. PMID: 12648728 Clinical Trial. - Metronidazole resistance in Helicobacter pylori.
Jenks PJ, Edwards DI. Jenks PJ, et al. Int J Antimicrob Agents. 2002 Jan;19(1):1-7. doi: 10.1016/s0924-8579(01)00468-x. Int J Antimicrob Agents. 2002. PMID: 11814762 Review. - Mechanism and clinical significance of metronidazole resistance in Helicobacter pylori.
van der Wouden EJ, Thijs JC, Kusters JG, van Zwet AA, Kleibeuker JH. van der Wouden EJ, et al. Scand J Gastroenterol Suppl. 2001;(234):10-4. doi: 10.1080/003655201753265055. Scand J Gastroenterol Suppl. 2001. PMID: 11768554 Review.
Cited by
- Introducing the NUATEI Consortium: A Mexican Research Program for the Identification of Natural and Synthetic Antimicrobial Compounds for Prevalent Infectious Diseases.
Carrero JC, Espinoza B, Huerta L, Silva-Miranda M, Guzmán-Gutierrez SL, Dorazco-González A, Reyes-Chilpa R, Espitia C, Sánchez S. Carrero JC, et al. Pharmaceuticals (Basel). 2024 Jul 18;17(7):957. doi: 10.3390/ph17070957. Pharmaceuticals (Basel). 2024. PMID: 39065807 Free PMC article. - Chemoprophylactic activity of nitazoxanide in experimental model of mammary gland carcinoma in rats.
Pal AK, Nandave M, Kaithwas G. Pal AK, et al. 3 Biotech. 2020 Aug;10(8):338. doi: 10.1007/s13205-020-02332-z. Epub 2020 Jul 8. 3 Biotech. 2020. PMID: 32670738 Free PMC article. - Nitazoxanide inhibits biofilm production and hemagglutination by enteroaggregative Escherichia coli strains by blocking assembly of AafA fimbriae.
Shamir ER, Warthan M, Brown SP, Nataro JP, Guerrant RL, Hoffman PS. Shamir ER, et al. Antimicrob Agents Chemother. 2010 Apr;54(4):1526-33. doi: 10.1128/AAC.01279-09. Epub 2010 Jan 19. Antimicrob Agents Chemother. 2010. PMID: 20086145 Free PMC article. - Nitazoxanide in treatment of Helicobacter pylori: a clinical and in vitro study.
Guttner Y, Windsor HM, Viiala CH, Dusci L, Marshall BJ. Guttner Y, et al. Antimicrob Agents Chemother. 2003 Dec;47(12):3780-3. doi: 10.1128/AAC.47.12.3780-3783.2003. Antimicrob Agents Chemother. 2003. PMID: 14638482 Free PMC article. Clinical Trial. - Who's Winning the War? Molecular Mechanisms of Antibiotic Resistance in Helicobacter pylori.
Jones KR, Cha JH, Merrell DS. Jones KR, et al. Curr Drug ther. 2008 Sep 1;3(3):190-203. doi: 10.2174/157488508785747899. Curr Drug ther. 2008. PMID: 21765819 Free PMC article.
References
- Broekhuysen, J., A. Stockis, R. L. Lins, J. De Graeve, and J. F. Rossignol. 2000. Nitazoxanide: pharmacokinetics and metabolism in man. Int. J. Clin. Pharmacol. Ther. 38:387-394. - PubMed
- Bryant, C., and M. DeLuca. 1991. Purification and characterization of an oxygen-insensitive NAD(P)H nitroreductase from Enterobacter cloacae. J. Biol. Chem. 266:4119-4125. - PubMed
- Chalker, A. F., H. W. Minehart, N. J. Hughes, K. K. Koretke, M. A. Lonetto, K. K. Brinkman, P. V. Warren, A. Lupas, M. J. Stanhope, J. R. Brown, and P. S. Hoffman. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 183:1259-1268. - PMC - PubMed
- Doumbo, O., J. F. Rossignol, E. Pichard, H. A. Traore, T. M. Dembele, M. Diakite, F. Traore, and D. A. Diallo. 1997. Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am. J. Trop. Med. Hyg. 56:637-639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources