Sequential SNARE disassembly and GATE-16-GOS-28 complex assembly mediated by distinct NSF activities drives Golgi membrane fusion - PubMed (original) (raw)
Comparative Study
. 2002 Jun 24;157(7):1161-73.
doi: 10.1083/jcb.200202082. Epub 2002 Jun 17.
Affiliations
- PMID: 12070132
- PMCID: PMC2173554
- DOI: 10.1083/jcb.200202082
Comparative Study
Sequential SNARE disassembly and GATE-16-GOS-28 complex assembly mediated by distinct NSF activities drives Golgi membrane fusion
Joyce M M Muller et al. J Cell Biol. 2002.
Abstract
Characterization of mammalian NSF (G274E) and Drosophila NSF (comatose) mutants revealed an evolutionarily conserved NSF activity distinct from ATPase-dependent SNARE disassembly that was essential for Golgi membrane fusion. Analysis of mammalian NSF function during cell-free assembly of Golgi cisternae from mitotic Golgi fragments revealed that NSF disassembles Golgi SNAREs during mitotic Golgi fragmentation. A subsequent ATPase-independent NSF activity restricted to the reassembly phase is essential for membrane fusion. NSF/alpha-SNAP catalyze the binding of GATE-16 to GOS-28, a Golgi v-SNARE, in a manner that requires ATP but not ATP hydrolysis. GATE-16 is essential for NSF-driven Golgi reassembly and precludes GOS-28 from binding to its cognate t-SNARE, syntaxin-5. We suggest that this occurs at the inception of Golgi reassembly to protect the v-SNARE and regulate SNARE function.
Figures
Figure 1.
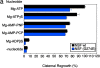
SNARE disassembly is not required during the reassembly of MGFs. (a) NSF proteins were added to a mixture of NEM-treated MGFs, α-SNAP, γ-SNAP, and p115 and incubated in the presence of the indicated nucleotides for 1 h at 25°C. Membranes were processed for EM, and the amount of cisternal regrowth was determined. Values represent means ± SEM (n = 3). (b and c) Increasing amounts of NSF proteins were added to reactions as in panel a in the presence of ATP (b) or ATPγS (c) and incubated for 1 h at 25°C. Reactions were processed as in panel a. Values represent means ± SEM (n = 3). (d) The ATPase activity of NSF (wt and mutant) plus or minus α-SNAP was measured by the release of [γ-32P] from [γ-32P]ATP or [γ-35S] from [γ-35S]ATPγS at 25°C. (e) RLGs were incubated with buffer or mitotic cytosol at 37°C for 30 min, reisolated, and resuspended in SDS sample buffer. Samples were incubated at 37 or 100°C before electrophoresis and analyzed for the presence of high molecular weight complexes containing syntaxin-5, rbet-1, or Ykt6 by immunoblot (IB). Arrows indicate high molecular weight SDS- resistant complexes.
Figure 1.
SNARE disassembly is not required during the reassembly of MGFs. (a) NSF proteins were added to a mixture of NEM-treated MGFs, α-SNAP, γ-SNAP, and p115 and incubated in the presence of the indicated nucleotides for 1 h at 25°C. Membranes were processed for EM, and the amount of cisternal regrowth was determined. Values represent means ± SEM (n = 3). (b and c) Increasing amounts of NSF proteins were added to reactions as in panel a in the presence of ATP (b) or ATPγS (c) and incubated for 1 h at 25°C. Reactions were processed as in panel a. Values represent means ± SEM (n = 3). (d) The ATPase activity of NSF (wt and mutant) plus or minus α-SNAP was measured by the release of [γ-32P] from [γ-32P]ATP or [γ-35S] from [γ-35S]ATPγS at 25°C. (e) RLGs were incubated with buffer or mitotic cytosol at 37°C for 30 min, reisolated, and resuspended in SDS sample buffer. Samples were incubated at 37 or 100°C before electrophoresis and analyzed for the presence of high molecular weight complexes containing syntaxin-5, rbet-1, or Ykt6 by immunoblot (IB). Arrows indicate high molecular weight SDS- resistant complexes.
Figure 2.
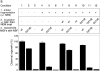
Two NSF activities are required for the fusion of MGFs. (a) Fragmentation reactions were pretreated with NEM for 15 min on ice followed by DTT for 15 min on ice (conditions 4–12). NEM prequenched with DTT served as the control (conditions 1–3). NSF (wt or mutant) and α-SNAP (conditions 7–12) or buffer (conditions 1–6) was then added, and reactions were incubated for 10 min at 25°C. NSF was then inactivated with NEM, and the reaction continued for 20 min at 37°C. MGFs were isolated and incubated in standard fusion assays with NSF (wt or mutant) or buffer at 25°C. Reactions were processed for EM, and the extent of cisternal regrowth was determined. Values represent means ± SEM (n = 3). (b) Fragmentation reactions were performed as in panel a except that MGFs were solubilized with Triton X-100 buffer. RLGs incubated with buffer instead of mitotic cytosol served as the control (lane 5). GOS-28 or GS15 was then immunoprecipitated. Immunocomplexes were analyzed for the presence of syntaxin-5, GOS-28, and GS15 by immunoblot.
Figure 2.
Two NSF activities are required for the fusion of MGFs. (a) Fragmentation reactions were pretreated with NEM for 15 min on ice followed by DTT for 15 min on ice (conditions 4–12). NEM prequenched with DTT served as the control (conditions 1–3). NSF (wt or mutant) and α-SNAP (conditions 7–12) or buffer (conditions 1–6) was then added, and reactions were incubated for 10 min at 25°C. NSF was then inactivated with NEM, and the reaction continued for 20 min at 37°C. MGFs were isolated and incubated in standard fusion assays with NSF (wt or mutant) or buffer at 25°C. Reactions were processed for EM, and the extent of cisternal regrowth was determined. Values represent means ± SEM (n = 3). (b) Fragmentation reactions were performed as in panel a except that MGFs were solubilized with Triton X-100 buffer. RLGs incubated with buffer instead of mitotic cytosol served as the control (lane 5). GOS-28 or GS15 was then immunoprecipitated. Immunocomplexes were analyzed for the presence of syntaxin-5, GOS-28, and GS15 by immunoblot.
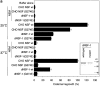
Figure 3.
The ATPase-independent activity of NSF is evolutionarily conserved. (a) Golgi reassembly was performed with the indicated NSF proteins (Drosophila or CHO) at 25 or 37°C. NSF proteins preincubated with NEM served as negative control. Reactions were processed for EM, and the amount of cisternal regrowth was determined. Values represent means ± SEM (n = 2). SDS-PAGE analysis of the pure His6-tagged dNSF proteins is shown in the inset. (b) Comatose dNSF-1 irreversibly changes conformation at 37°C. dNSF-1 proteins were incubated in the presence of Mg-ATP for 90 min at 25 or 37°C. After 45 min, one set of the 37°C samples was shifted to 25°C. Samples were processed for negative staining. A representative set of three end-on views is shown on the left, together with an averaged (n = 300) and sixfold symmetrized view on the right. (c) dNSF-1 proteins were incubated with Mg-ATP γS or Mg-ADP for 45 min at 4°C. Samples were processed and analyzed as in panel b. Note that Mg-ATPγS increases the diameter of wt dNSF-1 but not mutant dNSF-1 barrel from ∼12 to 15 nm. (d) NEM-treated light membranes isolated from fly heads were incubated with dSNAP and dNSF-1 (wt or mutant) in Mg-ATP for 40 min at 25°C. SDS sample buffer was then added, and the presence of high molecular weight complexes containing dsyntaxin-1A was determined by immunoblot.
Figure 3.
The ATPase-independent activity of NSF is evolutionarily conserved. (a) Golgi reassembly was performed with the indicated NSF proteins (Drosophila or CHO) at 25 or 37°C. NSF proteins preincubated with NEM served as negative control. Reactions were processed for EM, and the amount of cisternal regrowth was determined. Values represent means ± SEM (n = 2). SDS-PAGE analysis of the pure His6-tagged dNSF proteins is shown in the inset. (b) Comatose dNSF-1 irreversibly changes conformation at 37°C. dNSF-1 proteins were incubated in the presence of Mg-ATP for 90 min at 25 or 37°C. After 45 min, one set of the 37°C samples was shifted to 25°C. Samples were processed for negative staining. A representative set of three end-on views is shown on the left, together with an averaged (n = 300) and sixfold symmetrized view on the right. (c) dNSF-1 proteins were incubated with Mg-ATP γS or Mg-ADP for 45 min at 4°C. Samples were processed and analyzed as in panel b. Note that Mg-ATPγS increases the diameter of wt dNSF-1 but not mutant dNSF-1 barrel from ∼12 to 15 nm. (d) NEM-treated light membranes isolated from fly heads were incubated with dSNAP and dNSF-1 (wt or mutant) in Mg-ATP for 40 min at 25°C. SDS sample buffer was then added, and the presence of high molecular weight complexes containing dsyntaxin-1A was determined by immunoblot.
Figure 3.
The ATPase-independent activity of NSF is evolutionarily conserved. (a) Golgi reassembly was performed with the indicated NSF proteins (Drosophila or CHO) at 25 or 37°C. NSF proteins preincubated with NEM served as negative control. Reactions were processed for EM, and the amount of cisternal regrowth was determined. Values represent means ± SEM (n = 2). SDS-PAGE analysis of the pure His6-tagged dNSF proteins is shown in the inset. (b) Comatose dNSF-1 irreversibly changes conformation at 37°C. dNSF-1 proteins were incubated in the presence of Mg-ATP for 90 min at 25 or 37°C. After 45 min, one set of the 37°C samples was shifted to 25°C. Samples were processed for negative staining. A representative set of three end-on views is shown on the left, together with an averaged (n = 300) and sixfold symmetrized view on the right. (c) dNSF-1 proteins were incubated with Mg-ATP γS or Mg-ADP for 45 min at 4°C. Samples were processed and analyzed as in panel b. Note that Mg-ATPγS increases the diameter of wt dNSF-1 but not mutant dNSF-1 barrel from ∼12 to 15 nm. (d) NEM-treated light membranes isolated from fly heads were incubated with dSNAP and dNSF-1 (wt or mutant) in Mg-ATP for 40 min at 25°C. SDS sample buffer was then added, and the presence of high molecular weight complexes containing dsyntaxin-1A was determined by immunoblot.
Figure 3.
The ATPase-independent activity of NSF is evolutionarily conserved. (a) Golgi reassembly was performed with the indicated NSF proteins (Drosophila or CHO) at 25 or 37°C. NSF proteins preincubated with NEM served as negative control. Reactions were processed for EM, and the amount of cisternal regrowth was determined. Values represent means ± SEM (n = 2). SDS-PAGE analysis of the pure His6-tagged dNSF proteins is shown in the inset. (b) Comatose dNSF-1 irreversibly changes conformation at 37°C. dNSF-1 proteins were incubated in the presence of Mg-ATP for 90 min at 25 or 37°C. After 45 min, one set of the 37°C samples was shifted to 25°C. Samples were processed for negative staining. A representative set of three end-on views is shown on the left, together with an averaged (n = 300) and sixfold symmetrized view on the right. (c) dNSF-1 proteins were incubated with Mg-ATP γS or Mg-ADP for 45 min at 4°C. Samples were processed and analyzed as in panel b. Note that Mg-ATPγS increases the diameter of wt dNSF-1 but not mutant dNSF-1 barrel from ∼12 to 15 nm. (d) NEM-treated light membranes isolated from fly heads were incubated with dSNAP and dNSF-1 (wt or mutant) in Mg-ATP for 40 min at 25°C. SDS sample buffer was then added, and the presence of high molecular weight complexes containing dsyntaxin-1A was determined by immunoblot.
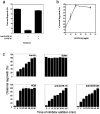
Figure 4.
GATE-16 is involved in Golgi reassembly. (a) Standard assays were performed with anti–GATE-16 antibodies and His6–GATE-16 protein as indicated, and the amount of cisternal regrowth was determined. Values represent means ± SEM (n = 3). (b) Increasing concentrations of His6–GATE-16 were added to standard assays. Values represent means ± SEM (n = 3). (C) Standard fusion assays were performed except that at the indicated times reactions were either terminated by fixation or treated with either buffer, NEM, anti–GOS-28 antibodies, or anti–GATE-16 antibodies after which the reaction was allowed to proceed for a total time of 1 h. Samples were analyzed as in panel a. Values represent means ± SEM (n = 4).
Figure 5.
GATE-16 regulates GOS-28–syntaxin-5 binding. (a) GATE-16 was mixed with RLG or MGF extract followed by the addition of anti–GOS-28 beads (IP). Immunocomplexes were isolated and analyzed for the presence of GOS-28, syntaxin-5, rbet1, and GATE-16 by immunoblot. (b) His6–GOS-28 (75 nM) was incubated for 1 h on ice with GST–syntaxin-5 (75 nM) and increasing concentrations of His6–GATE-16 (0–3,750 nM). GST–syntaxin-5 was retrieved with glutathione sepharose (top), or His6–GOS-28 was immunoprecipitated (bottom). Reactions were then processed for immunoblot.
Figure 6.
NSF/α-SNAP–dependent formation of GATE-16–GOS-28 complexes. (a) NSF (wt or mutant) was incubated with α-SNAP, His6–GATE-16, and MGF extract for 1 h on ice. GATE-16 was immunoprecipitated, and the extent of GOS-28, NSF, α-SNAP, and syntaxin-5 coprecipitation was determined by immunoblot. In some reactions, NSF (wt or mutant) was preincubated in the presence of Mg-ATP at 37°C for 30 min. In others, NSF (wt or mutant) was inactivated with NEM. (b) NSF wt or mutant (0.03 μM) was incubated with α-SNAP (0.1 μM), bio–GATE-16 (0.5 μM), and His6–GOS-28 (0.5 μM) in the presence of 2 mM Mg-ATP for 1 h on ice. bio–GATE-16 was retrieved with monomeric avidin beads. Washed beads were eluted with biotin, and eluates were fractionated by SDS-PAGE. The extent of GOS-28, α-SNAP, and NSF coprecipitation was determined by Coomassie staining. In some reactions, NSF (wt or mutant) was preincubated with Mg-ATP at 37°C for 30 min. In other reactions, NSF (wt or mutant) was inactivated with NEM. (c) The ATPase activity of NSF (wt and mutant) was measured by the release of [γ-32P] from [γ-32P]ATP plus or minus different combinations of GATE-16, α-SNAP, and GOS-28 at 25°C.
Figure 6.
NSF/α-SNAP–dependent formation of GATE-16–GOS-28 complexes. (a) NSF (wt or mutant) was incubated with α-SNAP, His6–GATE-16, and MGF extract for 1 h on ice. GATE-16 was immunoprecipitated, and the extent of GOS-28, NSF, α-SNAP, and syntaxin-5 coprecipitation was determined by immunoblot. In some reactions, NSF (wt or mutant) was preincubated in the presence of Mg-ATP at 37°C for 30 min. In others, NSF (wt or mutant) was inactivated with NEM. (b) NSF wt or mutant (0.03 μM) was incubated with α-SNAP (0.1 μM), bio–GATE-16 (0.5 μM), and His6–GOS-28 (0.5 μM) in the presence of 2 mM Mg-ATP for 1 h on ice. bio–GATE-16 was retrieved with monomeric avidin beads. Washed beads were eluted with biotin, and eluates were fractionated by SDS-PAGE. The extent of GOS-28, α-SNAP, and NSF coprecipitation was determined by Coomassie staining. In some reactions, NSF (wt or mutant) was preincubated with Mg-ATP at 37°C for 30 min. In other reactions, NSF (wt or mutant) was inactivated with NEM. (c) The ATPase activity of NSF (wt and mutant) was measured by the release of [γ-32P] from [γ-32P]ATP plus or minus different combinations of GATE-16, α-SNAP, and GOS-28 at 25°C.
Figure 6.
NSF/α-SNAP–dependent formation of GATE-16–GOS-28 complexes. (a) NSF (wt or mutant) was incubated with α-SNAP, His6–GATE-16, and MGF extract for 1 h on ice. GATE-16 was immunoprecipitated, and the extent of GOS-28, NSF, α-SNAP, and syntaxin-5 coprecipitation was determined by immunoblot. In some reactions, NSF (wt or mutant) was preincubated in the presence of Mg-ATP at 37°C for 30 min. In others, NSF (wt or mutant) was inactivated with NEM. (b) NSF wt or mutant (0.03 μM) was incubated with α-SNAP (0.1 μM), bio–GATE-16 (0.5 μM), and His6–GOS-28 (0.5 μM) in the presence of 2 mM Mg-ATP for 1 h on ice. bio–GATE-16 was retrieved with monomeric avidin beads. Washed beads were eluted with biotin, and eluates were fractionated by SDS-PAGE. The extent of GOS-28, α-SNAP, and NSF coprecipitation was determined by Coomassie staining. In some reactions, NSF (wt or mutant) was preincubated with Mg-ATP at 37°C for 30 min. In other reactions, NSF (wt or mutant) was inactivated with NEM. (c) The ATPase activity of NSF (wt and mutant) was measured by the release of [γ-32P] from [γ-32P]ATP plus or minus different combinations of GATE-16, α-SNAP, and GOS-28 at 25°C.
Figure 7.
NSF/α-SNAP–stimulated GOS-28–GATE-16 binding is nucleotide dependent. (a) NSF (wt or mutant) was incubated with α-SNAP, bio–GATE-16, and MGF extract for 1 h on ice plus either Mg-ATP, Mg-ATPγS, or Mg-ADPβS. bio–GATE-16 was retrieved with monomeric avidin beads, and the extent of GOS-28 and NSF coprecipitation was determined by immunoblot. (b and c) NSF wt (b) or mutant (c) (0.03 μM) was incubated for 1 h on ice with α-SNAP (0.1 μM), bio–GATE-16 (0.5 μM), and His6–GOS-28 (0.5 μM) plus either 2 mM Mg-ATP, Mg-ATPγS, Mg-AMP-PNP, Mg-AMP-PCP, Mg-ADP, Mg-ADPβS, or no nucleotide. bio–GATE-16 was retrieved with monomeric avidin beads. Washed beads were eluted with biotin and eluates fractionated by SDS-PAGE. The extent of GOS-28, α-SNAP, and NSF coprecipitation was determined by Coomassie staining.
Figure 7.
NSF/α-SNAP–stimulated GOS-28–GATE-16 binding is nucleotide dependent. (a) NSF (wt or mutant) was incubated with α-SNAP, bio–GATE-16, and MGF extract for 1 h on ice plus either Mg-ATP, Mg-ATPγS, or Mg-ADPβS. bio–GATE-16 was retrieved with monomeric avidin beads, and the extent of GOS-28 and NSF coprecipitation was determined by immunoblot. (b and c) NSF wt (b) or mutant (c) (0.03 μM) was incubated for 1 h on ice with α-SNAP (0.1 μM), bio–GATE-16 (0.5 μM), and His6–GOS-28 (0.5 μM) plus either 2 mM Mg-ATP, Mg-ATPγS, Mg-AMP-PNP, Mg-AMP-PCP, Mg-ADP, Mg-ADPβS, or no nucleotide. bio–GATE-16 was retrieved with monomeric avidin beads. Washed beads were eluted with biotin and eluates fractionated by SDS-PAGE. The extent of GOS-28, α-SNAP, and NSF coprecipitation was determined by Coomassie staining.
Figure 7.
NSF/α-SNAP–stimulated GOS-28–GATE-16 binding is nucleotide dependent. (a) NSF (wt or mutant) was incubated with α-SNAP, bio–GATE-16, and MGF extract for 1 h on ice plus either Mg-ATP, Mg-ATPγS, or Mg-ADPβS. bio–GATE-16 was retrieved with monomeric avidin beads, and the extent of GOS-28 and NSF coprecipitation was determined by immunoblot. (b and c) NSF wt (b) or mutant (c) (0.03 μM) was incubated for 1 h on ice with α-SNAP (0.1 μM), bio–GATE-16 (0.5 μM), and His6–GOS-28 (0.5 μM) plus either 2 mM Mg-ATP, Mg-ATPγS, Mg-AMP-PNP, Mg-AMP-PCP, Mg-ADP, Mg-ADPβS, or no nucleotide. bio–GATE-16 was retrieved with monomeric avidin beads. Washed beads were eluted with biotin and eluates fractionated by SDS-PAGE. The extent of GOS-28, α-SNAP, and NSF coprecipitation was determined by Coomassie staining.
Figure 8.
NSF/α-SNAP catalyze the formation of GATE-16–GOS-28 complexes in the absence of ATP hydrolysis. (a–d) NSF wt (5 nM; a and c) or NSF G274E (5 nM; b and d) was incubated with α-SNAP (0.1 μM), His6–GOS-28 (0.5 μM), and bio–GATE-16 avidin beads in the presence of 2 mM Mg-ATP (a and b) or 2 mM Mg-AMP-PCP (c and d) for 2 min–2 h on ice. At the indicated time points, beads were recovered. Washed beads were eluted, and eluates were fractionated by SDS-PAGE. The extent of GOS-28, α-SNAP, and NSF coprecipitation was determined by Coomassie staining.
Similar articles
- An NSF function distinct from ATPase-dependent SNARE disassembly is essential for Golgi membrane fusion.
Müller JM, Rabouille C, Newman R, Shorter J, Freemont P, Schiavo G, Warren G, Shima DT. Müller JM, et al. Nat Cell Biol. 1999 Oct;1(6):335-40. doi: 10.1038/14025. Nat Cell Biol. 1999. PMID: 10559959 - GATE-16, a membrane transport modulator, interacts with NSF and the Golgi v-SNARE GOS-28.
Sagiv Y, Legesse-Miller A, Porat A, Elazar Z. Sagiv Y, et al. EMBO J. 2000 Apr 3;19(7):1494-504. doi: 10.1093/emboj/19.7.1494. EMBO J. 2000. PMID: 10747018 Free PMC article. - Biochemical analysis of the Saccharomyces cerevisiae SEC18 gene product: implications for the molecular mechanism of membrane fusion.
Steel GJ, Laude AJ, Boojawan A, Harvey DJ, Morgan A. Steel GJ, et al. Biochemistry. 1999 Jun 15;38(24):7764-72. doi: 10.1021/bi990315v. Biochemistry. 1999. PMID: 10387016 - SNAREs and membrane fusion in the Golgi apparatus.
Nichols BJ, Pelham HR. Nichols BJ, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review. - Fusion of membranes during the acrosome reaction: a tale of two SNAREs.
Kierszenbaum AL. Kierszenbaum AL. Mol Reprod Dev. 2000 Dec;57(4):309-10. doi: 10.1002/1098-2795(200012)57:4<309::AID-MRD1>3.0.CO;2-W. Mol Reprod Dev. 2000. PMID: 11066058 Review.
Cited by
- Mammalian Atg8 proteins regulate lysosome and autolysosome biogenesis through SNAREs.
Gu Y, Princely Abudu Y, Kumar S, Bissa B, Choi SW, Jia J, Lazarou M, Eskelinen EL, Johansen T, Deretic V. Gu Y, et al. EMBO J. 2019 Nov 15;38(22):e101994. doi: 10.15252/embj.2019101994. Epub 2019 Oct 18. EMBO J. 2019. PMID: 31625181 Free PMC article. - VCIP135, a novel essential factor for p97/p47-mediated membrane fusion, is required for Golgi and ER assembly in vivo.
Uchiyama K, Jokitalo E, Kano F, Murata M, Zhang X, Canas B, Newman R, Rabouille C, Pappin D, Freemont P, Kondo H. Uchiyama K, et al. J Cell Biol. 2002 Dec 9;159(5):855-66. doi: 10.1083/jcb.200208112. Epub 2002 Dec 9. J Cell Biol. 2002. PMID: 12473691 Free PMC article. - Unlocking the gate to GABARAPL2.
Chan JCY, Gorski SM. Chan JCY, et al. Biol Futur. 2022 Jun;73(2):157-169. doi: 10.1007/s42977-022-00119-2. Epub 2022 Apr 29. Biol Futur. 2022. PMID: 35486231 Review. - An atypical LIR motif within UBA5 (ubiquitin like modifier activating enzyme 5) interacts with GABARAP proteins and mediates membrane localization of UBA5.
Huber J, Obata M, Gruber J, Akutsu M, Löhr F, Rogova N, Güntert P, Dikic I, Kirkin V, Komatsu M, Dötsch V, Rogov VV. Huber J, et al. Autophagy. 2020 Feb;16(2):256-270. doi: 10.1080/15548627.2019.1606637. Epub 2019 Apr 28. Autophagy. 2020. PMID: 30990354 Free PMC article. - Atg8 family proteins, LIR/AIM motifs and other interaction modes.
Rogov VV, Nezis IP, Tsapras P, Zhang H, Dagdas Y, Noda NN, Nakatogawa H, Wirth M, Mouilleron S, McEwan DG, Behrends C, Deretic V, Elazar Z, Tooze SA, Dikic I, Lamark T, Johansen T. Rogov VV, et al. Autophagy Rep. 2023 Mar 19;2(1):27694127.2023.2188523. doi: 10.1080/27694127.2023.2188523. eCollection 2023 Dec 31. Autophagy Rep. 2023. PMID: 38214012 Free PMC article.
References
- Banerjee, A., V.A. Barry, B.R. DasGupta, and T.F.J. Martin. 1996. NSF acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 271:20223–20226. - PubMed
- Chen, Y.A., S.J. Scales, S.M. Patel, Y.C. Doung, and R.H. Scheller. 1999. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 97:165–174. - PubMed
- Colombo, M.I., M. Taddese, S.W. Whiteheart, and P.D. Stahl. 1996. A possible predocking attachment site for NSF. Insights from in vitro endosome fusion. J. Biol. Chem. 271:18810–18816. - PubMed
- Fisher, R.J., J. Pevsner, and R.D. Burgoyne. 2001. Control of fusion pore dynamics during exocytosis by Munc18. Science. 291:875–878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases