Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming - PubMed (original) (raw)
Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming
Frederique Varoqueaux et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Synaptic vesicles must be primed to fusion competence before they can fuse with the plasma membrane in response to increased intracellular Ca2+ levels. The presynaptic active zone protein Munc13-1 is essential for priming of glutamatergic synaptic vesicles in hippocampal neurons. However, a small subpopulation of synapses in any given glutamatergic nerve cell as well as all gamma-aminobutyratergic (GABAergic) synapses are largely independent of Munc13-1. We show here that Munc13-2, the only Munc13 isoform coexpressed with Munc13-1 in hippocampus, is responsible for vesicle priming in Munc13-1 independent hippocampal synapses. Neurons lacking both Munc13-1 and Munc13-2 show neither evoked nor spontaneous release events, yet form normal numbers of synapses with typical ultrastructural features. Thus, the two Munc13 isoforms are completely redundant in GABAergic cells whereas glutamatergic neurons form two types of synapses, one of which is solely Munc13-1 dependent and lacks Munc13-2 whereas the other type employs Munc13-2 as priming factor. We conclude that Munc13-mediated vesicle priming is not a transmitter specific phenomenon but rather a general and essential feature of multiple fast neurotransmitter systems, and that synaptogenesis during development is not dependent on synaptic secretory activity.
Figures
Figure 1
Mutation of the murine _Munc13_-2 gene. (A) Maps of the wild-type _Munc13_-2 gene, the respective targeting vector, and the resulting mutant gene. Positions of exons (black boxes) and restriction enzyme sites are indicated. Hatched bar, Position of probe used for Southern. Neo, Neomycin resistance gene; TK, thymidine kinase gene. (B) Southern blot analysis of the Munc13-2 KO mutations in mice by using mouse tail DNA (_Eco_RI digest) from the indicated Munc13-2 genotypes. (C) Munc13-2 protein expression in mice of the indicated genotypes as determined by Western blotting using an antibody that recognizes all Munc13-2 splice variants. (D) Expression of Munc13-1 and Munc13-2 protein in mice of the indicated genotypes as determined by Western blotting using an antibody to Munc13-1 (Upper) and an antibody that recognizes all Munc13-2 splice variants (Lower).
Figure 2
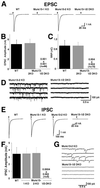
Complex functional redundancy of Munc13 isoforms. (A) Examples of EPSCs recorded from cells of the indicated genotypes. (B) Mean EPSC amplitudes measured in cells of the indicated genotypes. (C) Mean readily releasable vesicle pool sizes in cells of the indicated genotypes as estimated by the charge integral measured after release induced by application of 500 mOsm hypertonic sucrose solution for 4 s. (D) mEPSC activity recorded in cells of the indicated genotypes. Holding potential −70 mV. (E) Examples of IPSCs recorded from cells of the indicated genotypes. Munc13-1/2 DKO inhibitory neurons were identified after each experiment by detection of mIPSC events induced by application of α-latrotoxin. (F) Mean IPSC amplitudes measured in cells of the indicated genotypes. (G) mIPSC activity recorded in cells of the indicated genotypes. Pipette solution contained 140 mM KCl.
Figure 3
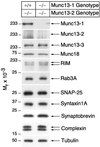
Normal expression of synaptic proteins in Munc13-1/2 DKO brains. Brain homogenates of the indicated genotypes were analyzed by Western blotting using specific antibodies to the indicated proteins.
Figure 4
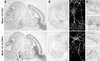
Normal morphology of Munc13-1/2 DKO brains and cultured cells. (A) Nissl-stained sagittal section of brains from E18 mice of the indicated genotype. (Bar = 500 μm.) (B) Nissl-stained coronal sections of spinal cord from E18 mice of the indicated genotype. (Bar = 100 μm.) (C) Cultured hippocampal neurons from E18 mice of the indicated genotype (12 DIV) stained for the synapse specific marker synaptophysin. (Bar = 10 μm.) (D) Electron micrographs of synapses of the indicated genotypes. (Bar = 100 nm.)
Figure 5
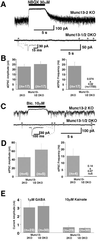
Normal postsynaptic responsiveness in release-incompetent Munc 13-1/2 double-deficient neurons. (A) Examples of mEPSC activity in cells of the indicated genotypes after application of 1 nM α-latrotoxin for 1 min. Note that signals are blocked by 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[_f_]quinoxaline (NBQX). Stars indicate mEPSC events. (Inset) Individual mEPSC (expanded scale). (B) Average mEPSC amplitude and frequency in cells of the indicated genotypes after treatment with 1 nM α-latrotoxin. (C) Examples of mIPSC activity in cells of the indicated genotypes after application of 1 nM α-latrotoxin for 1 min. Note that signals are blocked by bicuculline. Stars indicate mIPSC events. Inset shows individual mIPSC (expanded scale). (D) Average mIPSC amplitude and frequency in cells of the indicated genotypes after treatment with 1 nM α-latrotoxin. (E) Mean peak current responses evoked by exogenous application of GABA and kainate recorded from cells of the indicated genotypes.
Figure 6
No endocytosis in release-incompetent Munc13-1/2 DKO neurons. Localization of endocytotically active synapses by activity-dependent FM1-43 staining in cells of the indicated genotypes. Images show cells after staining and wash. (Bar = 10 μm.)
Similar articles
- Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles.
Augustin I, Rosenmund C, Südhof TC, Brose N. Augustin I, et al. Nature. 1999 Jul 29;400(6743):457-61. doi: 10.1038/22768. Nature. 1999. PMID: 10440375 - A common molecular basis for membrane docking and functional priming of synaptic vesicles.
Siksou L, Varoqueaux F, Pascual O, Triller A, Brose N, Marty S. Siksou L, et al. Eur J Neurosci. 2009 Jul;30(1):49-56. doi: 10.1111/j.1460-9568.2009.06811.x. Epub 2009 Jun 25. Eur J Neurosci. 2009. PMID: 19558619 - Identification of the minimal protein domain required for priming activity of Munc13-1.
Stevens DR, Wu ZX, Matti U, Junge HJ, Schirra C, Becherer U, Wojcik SM, Brose N, Rettig J. Stevens DR, et al. Curr Biol. 2005 Dec 20;15(24):2243-8. doi: 10.1016/j.cub.2005.10.055. Epub 2005 Nov 3. Curr Biol. 2005. PMID: 16271475 - The synaptic vesicle cluster: a source of endocytic proteins during neurotransmitter release.
Shupliakov O. Shupliakov O. Neuroscience. 2009 Jan 12;158(1):204-10. doi: 10.1016/j.neuroscience.2008.03.035. Epub 2008 Mar 26. Neuroscience. 2009. PMID: 18440714 Review. - Information processing by graded-potential transmission through tonically active synapses.
Juusola M, French AS, Uusitalo RO, Weckström M. Juusola M, et al. Trends Neurosci. 1996 Jul;19(7):292-7. doi: 10.1016/S0166-2236(96)10028-X. Trends Neurosci. 1996. PMID: 8799975 Review.
Cited by
- Linkage of Alzheimer disease families with Puerto Rican ancestry identifies a chromosome 9 locus.
Rajabli F, Feliciano-Astacio BE, Cukier HN, Wang L, Griswold AJ, Hamilton-Nelson KL, Adams LD, Rodriguez VC, Mena PR, Tejada S, Celis K, Whitehead PL, Van Booven DJ, Hofmann NK, Bussies PL, Prough M, Chinea A, Feliciano NI, Vardarajan BN, Reitz C, Lee JH, Prince MJ, Jimenez IZ, Mayeux RP, Acosta H, Dalgard CL, Haines JL, Vance JM, Cuccaro ML, Beecham GW, Pericak-Vance MA. Rajabli F, et al. Neurobiol Aging. 2021 Aug;104:115.e1-115.e7. doi: 10.1016/j.neurobiolaging.2021.02.019. Epub 2021 Feb 28. Neurobiol Aging. 2021. PMID: 33902942 Free PMC article. - Fusion Competent Synaptic Vesicles Persist upon Active Zone Disruption and Loss of Vesicle Docking.
Wang SSH, Held RG, Wong MY, Liu C, Karakhanyan A, Kaeser PS. Wang SSH, et al. Neuron. 2016 Aug 17;91(4):777-791. doi: 10.1016/j.neuron.2016.07.005. Neuron. 2016. PMID: 27537483 Free PMC article. - Loss of MUNC13-1 function causes microcephaly, cortical hyperexcitability, and fatal myasthenia.
Engel AG, Selcen D, Shen XM, Milone M, Harper CM. Engel AG, et al. Neurol Genet. 2016 Sep 8;2(5):e105. doi: 10.1212/NXG.0000000000000105. eCollection 2016 Oct. Neurol Genet. 2016. PMID: 27648472 Free PMC article. - Building a synapse: a complex matter.
Kim YJ, Serpe M. Kim YJ, et al. Fly (Austin). 2013 Jul-Sep;7(3):146-52. doi: 10.4161/fly.24413. Epub 2013 Apr 8. Fly (Austin). 2013. PMID: 23680998 Free PMC article. - Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release.
Ma C, Su L, Seven AB, Xu Y, Rizo J. Ma C, et al. Science. 2013 Jan 25;339(6118):421-5. doi: 10.1126/science.1230473. Epub 2012 Dec 20. Science. 2013. PMID: 23258414 Free PMC article.
References
- Südhof T C. Nature (London) 1995;375:645–653. - PubMed
- Zucker R S. Neuron. 1996;17:1049–1055. - PubMed
- Augustin I, Rosenmund C, Südhof T C, Brose N. Nature (London) 1999b;400:457–461. - PubMed
- Aravamudan B, Fergestad T, Davis W S, Rodesch C K, Broadie K. Nat Neurosci. 1999;2:965–971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous