Subcellular distribution of Wnt pathway proteins in normal and neoplastic colon - PubMed (original) (raw)
Subcellular distribution of Wnt pathway proteins in normal and neoplastic colon
Christine B Anderson et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Mutations in the APC tumor suppressor gene are present in approximately 85% of colorectal tumors and are thought to contribute early in the process of tumorigenesis. The truncated protein resulting from most APC mutations can lead to elevated beta-catenin levels in colon tumor cells. APC and associated proteins thus form a beta-catenin regulatory complex, with axin playing a key role. Although cell culture studies have revealed intriguing aspects of this complex, little characterization has been done in human colonocytes, the target tissue of colon carcinogenesis. The present study of intact human colon crypts, adenomatous polyps, and adenocarcinomas focuses on subcellular localization of some key elements of the complex: beta-catenin, APC, axin, and axin2. We examined endogenous protein localization within the framework of three-dimensional tissue architecture by using laser scanning confocal microscopy, and immunofluorescence staining of whole-mount fixed tissue from more than 50 patients. Expression patterns suggest that APC and axin colocalize in the nucleus and at lateral cell borders, and show that axin2 is limited to the nucleus. Altered nuclear expression of axin seen in colon polyps and carcinomas may be a consequence of the loss of full-length APC and the advent of nuclear beta-catenin. The observation of nuclear beta-catenin in fewer than half of carcinoma images and only rarely in polyps indicates that nuclear translocation of beta-catenin may not be an immediate consequence of the loss of APC.
Figures
Figure 1
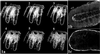
(a) Serial optical sections of mouse colon crypts labeled with the nuclear counterstain To-Pro 3. Sequential images along the _Z_-axis were collected using laser scanning confocal microscopy. Optical section 2 gives the clearest longitudinal section of the center crypt. To compare tissue preparation methods, colon tissue from a single patient was either fixed and the crypts manually microdissected (b), or treated with chelating agents to release the crypts, which were then fixed (c). Crypts were immunostained for APC and the protein localization compared. Crypts manipulated before fixation (c) exhibited altered protein localization.
Figure 2
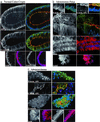
Expression of APC, β-catenin, axin, and axin2 in optical sections of whole-mount human colon tissue. The basic units of normal colonic mucosa, the crypts, are vase-shaped structures comprised of rectangular epithelial cells, stacked lengthwise. These cells have an apical side that faces the crypt lumen and a basal side that houses the nucleus. A grayscale image shows the primary antibody alone. Three-color merged images of APC or β-catenin show primary antibody in green, a nuclear counterstain in blue, and phalloidin-labeled actin in red, delineating a ring of adhesion proteins just below the apical cell membrane. The two-color merged images show axin or axin2 in red with a nuclear counter stain in blue. Arrows indicate apical surfaces and arrowheads mark nuclei. In normal crypts (a), APC and axin are found diffusely in the nucleus and near the borders between cells. APC is especially prominent near the apical junctions. β-catenin is found at lateral cell junctions and axin2 is nuclear. Adenomatous polyps (b) show strong nuclear N-terminal (truncated) APC, whereas C-terminal (full-length) APC and axin are missing or reduced in the nucleus. C-terminal APC is variably retained at cell–cell borders, and near the face of the apical membrane. Axin is strongly cytoplasmic. The β-catenin pattern is similar to that in normal crypts and axin2 remains nuclear. In carcinoma (c), expression patterns of the four proteins are similar to those in polyps except that β-catenin and axin are found in the nucleus. (Scale bar, 10 μm.)
Figure 3
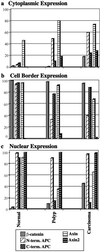
Graphic representation of data from Table 1, grouped by site of expression, cytoplasmic, cell border, or nuclear. In a, cytoplasmic expression of axin is seen in normal cells, but is found with greater frequency in polyp and carcinoma, as are cytoplasmic N-terminal (truncated) APC and axin2. Incidence of cytoplasmic β-catenin also rises slightly in carcinoma. In b, cell border expression of N-terminal APC is diminished in polyp and carcinoma. In c, nuclear expression of APC, axin, and axin2 is seen in normal cells. Nuclear C-terminal APC and axin are diminished in polyp, but nuclear axin is found again in carcinoma in conjunction with the expression of nuclear β-catenin.
Figure 4
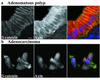
Double staining of β-catenin and axin in adenomatous polyp and adenocarcinoma. The polyp image (a) shows β-catenin and axin near cell–cell junctions, and axin strongly in the cytoplasm. Neither protein is obvious in the nucleus. The carcinoma image (b) shows both β-catenin and axin in the nucleus. Primary antibodies in each double-stained image are presented singly in grayscale, and in a three-color merged image showing β-catenin in green, axin in red, and nuclei in blue. (Scale bar, 10 μm.)
Similar articles
- AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1.
Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. Satoh S, et al. Nat Genet. 2000 Mar;24(3):245-50. doi: 10.1038/73448. Nat Genet. 2000. PMID: 10700176 - Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis.
Kolligs FT, Bommer G, Göke B. Kolligs FT, et al. Digestion. 2002;66(3):131-44. doi: 10.1159/000066755. Digestion. 2002. PMID: 12481159 Review. - Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors.
Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT. Yan D, et al. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):14973-8. doi: 10.1073/pnas.261574498. Proc Natl Acad Sci U S A. 2001. PMID: 11752446 Free PMC article. - Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level.
Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Nakamura T, et al. Genes Cells. 1998 Jun;3(6):395-403. doi: 10.1046/j.1365-2443.1998.00198.x. Genes Cells. 1998. PMID: 9734785 - The subcellular destinations of APC proteins.
Bienz M. Bienz M. Nat Rev Mol Cell Biol. 2002 May;3(5):328-38. doi: 10.1038/nrm806. Nat Rev Mol Cell Biol. 2002. PMID: 11988767 Review.
Cited by
- c-Cbl mediates the degradation of tumorigenic nuclear β-catenin contributing to the heterogeneity in Wnt activity in colorectal tumors.
Shashar M, Siwak J, Tapan U, Lee SY, Meyer RD, Parrack P, Tan J, Khatami F, Francis J, Zhao Q, Hartshorn K, Kolachalama VB, Rahimi N, Chitalia V. Shashar M, et al. Oncotarget. 2016 Nov 1;7(44):71136-71150. doi: 10.18632/oncotarget.12107. Oncotarget. 2016. PMID: 27661103 Free PMC article. - Adenomatous polyposis coli (APC) plays multiple roles in the intestinal and colorectal epithelia.
Senda T, Iizuka-Kogo A, Onouchi T, Shimomura A. Senda T, et al. Med Mol Morphol. 2007 Jun;40(2):68-81. doi: 10.1007/s00795-006-0352-5. Epub 2007 Jun 18. Med Mol Morphol. 2007. PMID: 17572842 Review. - Analysis of a panel of antibodies to APC reveals consistent activity towards an unidentified protein.
Davies ML, Roberts GT, Stuart N, Wakeman JA. Davies ML, et al. Br J Cancer. 2007 Aug 6;97(3):384-90. doi: 10.1038/sj.bjc.6603873. Epub 2007 Jun 26. Br J Cancer. 2007. PMID: 17595655 Free PMC article. - When You Come to a Fork in the Road, Take It: Wnt Signaling Activates Multiple Pathways through the APC/Axin/GSK-3 Complex.
Li C, Furth EE, Rustgi AK, Klein PS. Li C, et al. Cells. 2023 Sep 12;12(18):2256. doi: 10.3390/cells12182256. Cells. 2023. PMID: 37759479 Free PMC article. Review. - Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/β-catenin signaling.
Herbst A, Jurinovic V, Krebs S, Thieme SE, Blum H, Göke B, Kolligs FT. Herbst A, et al. BMC Genomics. 2014 Jan 28;15:74. doi: 10.1186/1471-2164-15-74. BMC Genomics. 2014. PMID: 24467841 Free PMC article.
References
- Fearnhead N S, Britton M P, Bodmer W F. Hum Mol Genet. 2001;10:721–733. - PubMed
- Junqueira L C, Carneiro J, Kelley R O. Basic Histology. Norwalk, CT: Appleton & Lange; 1992. p. 308.
- Hall P A, Coates P J, Ansari B, Hopwood D. J Cell Sci. 1994;107:3569–3577. - PubMed
- Marshman E, Booth C, Potten C S. BioEssays. 2002;24:91–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous