A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage - PubMed (original) (raw)
A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage
Kun Zou et al. J Neurosci. 2002.
Abstract
Aggregated and oligomeric amyloid beta-protein (Abeta) is known to exhibit neurotoxicity. However, the action of Abeta monomers on neurons is not fully understood. We have studied aggregation state-dependent actions of Abeta and found an oligomer-specific effect of Abeta on lipid metabolism in neurons (Michikawa et al., 2001). Here, we show a novel function of monomeric Abeta1-40, which is the major species found in physiological fluid, as a natural antioxidant molecule that prevents neuronal death caused by transition metal-induced oxidative damage. Monomeric Abeta1-40, which is demonstrated by SDS-PAGE after treatment with glutaraldehyde, protects neurons cultured in a medium containing 1.5 microm Fe(II) without antioxidant molecules. Metal ion chelators such as EDTA, CDTA (trans-1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid), and DTPA (diethylenetriamine-N,N,N',N",N"-penta-acetic acid, an iron-binding protein, transferrin, and antioxidant scavengers such as catalase, glutathione, and vitamin E also inhibit neuronal death under the same conditions. Monomeric Abeta1-40 inhibits neuronal death caused by Cu(II), Fe(II), and Fe(III) but does not protect neurons against H2O2-induced damage. Monomeric Abeta1-40 inhibits the reduction of Fe(III) induced by vitamin C and the generation of superoxides and prevents lipid peroxidation induced by Fe(II). Abeta1-42 remaining as a monomer also exhibits antioxidant and neuroprotective effects. In contrast, oligomeric and aggregated Abeta1-40 and Abeta1-42 lose their neuroprotective activity. These results indicate that monomeric Abeta protects neurons by quenching metal-inducible oxygen radical generation and thereby inhibiting neurotoxicity. Because aggregated Abeta is known to be an oxygen radical generator, our results provide a novel concept that the aggregation-dependent biological effects of Abeta are dualistic, being either an oxygen radical generator or its inhibitor.
Figures
Fig. 1.
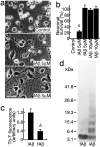
fAβ1–40 inhibits neuronal death induced in an antioxidant-depleted medium. Rat cortical neurons were cultured in the B27-AO medium without Aβ1–40 treatment: a, 40 hr culture; b, 48 hr culture;c, 64 hr culture; d, with fAβ1–40 (5 μ
m
) treatment 4 hr after plating, 64 hr culture.a_–_c are the same microscope field.e, Cell viability in the cultures treated with Aβ: none (●); DMSO vehicle (■); Aβ1–40 at 2 μ
m
(▪), 10 μ
m
(▵), and 20 μ
m
(○); and Aβ1–42 at 10 μ
m
(▴). Data represent means ± SE;n = 6 each. Six independent experiments showed similar results. Representative Hoechst staining showing the nuclear morphology of cortical neurons with or without Aβ1–40 treatment is shown in f, g. Cell membrane permeability is indicated by PI staining in h,i.
Fig. 2.
fAβ1–40 but not iAβ1–40 protects neurons in the B27-AO medium. Neurons were treated with fAβ1–40 or iAβ1–40 24 hr after plating. Phase-contrast photomicrographs were taken (a), and the cell viability was determined (b) 48 hr after the commencement of each treatment. a, p < 0.0001 versus fAβ1–40 (5 μ
m
), iAβ1–40 (10 μ
m
), and fAβ1–40 (10 μ
m
). c, Thioflavin-T fluorescence with the conditioned medium of each cultured neuron treated with fAβ1–40 or iAβ1–40 for 3 d. b, p < 0.01 versus iAβ1–40. d, Detection of oligomeric Aβ in fAβ and iAβ samples by cross-linking with glutaraldehyde. fAβ1–40 or iAβ1–40 (2.5 μg) was cross-linked with glutaraldehyde and subjected to a 4–20% SDS-PAGE. The gel was then visualized by silver staining.
Fig. 3.
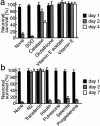
Transferrin and antioxidant scavengers inhibit neuronal death that occurred in the B27-AO medium. a, SOD (1500 U/ml), catalase (21,600 U/ml), glutathione (450 μg/ml), vitamin E acetate (1 μg/ml), or vitamin E (1 μg/ml) was added to neuronal cultures maintained in the B27-AO medium 4 hr after plating.b, N2 supplements or each component of N2 supplements, transferrin (100 μg/ml), insulin (5 μg/ml), progesterone (0.0063 μg/ml), putrescine (16.11 μg/ml), and selenite (0.0052 μg/ml) was added to neuronal cultures maintained in the B27-AO medium 4 hr after plating. Neuronal viability was determined as described in Materials and Methods at culture days 1, 2, and 4 (a) or 1, 2, and 7 (b).
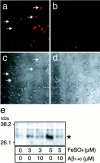
Fig. 4.
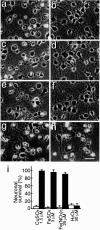
f Aβ1–40 inhibits neuronal death induced by transition-metal ions. Neurons were cultured in N2 medium for 24 hr, followed by treatment with 1.5 μ
m
CuSO4(a, b), 3.0 μ
m
Fe SO4 (c, d), 25 μ
m
Fe(NO3)3, (e, f), and 30 μ
m
H2O2 (g,h), and incubated for another 24 hr; photographs were taken to determine cell viability. a, c,e, and g represent control cultures, and_b_, d, f, and_h_ represent neurons treated with fAβ1–40 (5 μ
m
) in addition to metal ions. i, Quantitative analysis of these treatments 24 hr after the commencement of the metal treatment. Open and closed bars indicate cell viability in the cultures in the absence or presence, respectively, of fAβ1–40 (5 μ
m
).
Fig. 5.
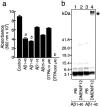
Inhibitory effect of Aβ peptides on vitamin C-mediated Fe(III) reduction and the aggregation state of Aβ peptides in PB and DMEM/F12. a, Aβ peptides were incubated with Fe(III) (25 μ
m
) and PDT (250 μ
m
), followed by the addition of vitamin C (10 μ
m
) and subsequent incubation for 14 hr at 37°C. The effects of freshly dissolved Aβ peptides (10 μ
m
) (fAβ1–40, fAβ1–42, fAβ1–16, and fAβ25–35, all of which were dissolved in distilled water to make a stock solution at 200 μ
m
) and DTPA (10 and 300 μ
m
) on the reduction of Fe(III) were determined. The amount of reduced iron ions was determined by measuring the absorbance at 562 nm. Data represent means ± SE; n = 5 replicate wells. p < 0.001 (a) and p < 0.0001 (b) versus control. b, Freshly dissolved Aβ1–40 and Aβ1–42 peptides at 20 μ
m
were incubated for 3 hr at 37°C in 8 m
m
sodium phosphate buffer, pH 7.4 (lanes 1, 3), or in DMEM/F12 medium (lanes 2, 4). The aggregation state of Aβ1–40 (lanes 1,2) and Aβ1–42 (lane 3,4) was visualized by SDS-PAGE and silver staining after cross-linking as described in Materials and Methods. Note that Aβ1–42 aggregated immediately in DMEM/F12 (∗), but the majority of both peptides, Aβ1–40 in both solutions and Aβ1–42 in PB, remained as a monomer.
Fig. 6.
fAβ1–40 inhibits the generation of superoxide and lipid peroxidation. Neurons were treated with (a,c) or without (b, d) 5 μ
m
fAβ1–40 4 hr after plating and were cultured for 48 hr in the B27-AO medium. The cultures were then incubated with 5 μ
m
HE fluorescence (a, b) for 30 min, and transmissive light micrographs of these cultures were taken. Note that the increased signal of superoxides was observed in swollen neurons (arrows) as well as in shrunken neurons in cultures without fAβ (a). e, The effect of Aβ on the production of lipid peroxidation in rat cerebral cortices in the presence of Fe(II). Cerebral cortices were isolated, minced with a cutter, and incubated in PBS in the presence of 3 and 5 μ
m
Fe(II) with or without fAβ1–40 at 10 μ
m
for 4 hr at 37°C. The fragments were then homogenized in RIPA buffer and centrifuged at 10,000 ×g for 10 min at 4°C. The supernatant of the homogenate was subjected to Western blot analysis using anti-4-HNE antibody as the primary antibody.
Similar articles
- Amyloid beta-protein (Abeta)1-40 protects neurons from damage induced by Abeta1-42 in culture and in rat brain.
Zou K, Kim D, Kakio A, Byun K, Gong JS, Kim J, Kim M, Sawamura N, Nishimoto S, Matsuzaki K, Lee B, Yanagisawa K, Michikawa M. Zou K, et al. J Neurochem. 2003 Nov;87(3):609-19. doi: 10.1046/j.1471-4159.2003.02018.x. J Neurochem. 2003. PMID: 14535944 - Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity.
Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Sultana R, et al. J Neurochem. 2005 Feb;92(4):749-58. doi: 10.1111/j.1471-4159.2004.02899.x. J Neurochem. 2005. PMID: 15686476 - Protective effects of vitamin E against oxidative damage induced by Abeta1-40Cu(II) complexes.
Dai X, Sun Y, Jiang Z. Dai X, et al. Acta Biochim Biophys Sin (Shanghai). 2007 Feb;39(2):123-30. doi: 10.1111/j.1745-7270.2007.00261.x. Acta Biochim Biophys Sin (Shanghai). 2007. PMID: 17277887 - Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review.
Butterfield DA. Butterfield DA. Free Radic Res. 2002 Dec;36(12):1307-13. doi: 10.1080/1071576021000049890. Free Radic Res. 2002. PMID: 12607822 Review. - The redox chemistry of the Alzheimer's disease amyloid beta peptide.
Smith DG, Cappai R, Barnham KJ. Smith DG, et al. Biochim Biophys Acta. 2007 Aug;1768(8):1976-90. doi: 10.1016/j.bbamem.2007.02.002. Epub 2007 Feb 9. Biochim Biophys Acta. 2007. PMID: 17433250 Review.
Cited by
- Tumor necrosis factor death receptor signaling cascade is required for amyloid-beta protein-induced neuron death.
Li R, Yang L, Lindholm K, Konishi Y, Yue X, Hampel H, Zhang D, Shen Y. Li R, et al. J Neurosci. 2004 Feb 18;24(7):1760-71. doi: 10.1523/JNEUROSCI.4580-03.2004. J Neurosci. 2004. PMID: 14973251 Free PMC article. - Dietary lipids in the aetiology of Alzheimer's disease: implications for therapy.
Cooper JL. Cooper JL. Drugs Aging. 2003;20(6):399-418. doi: 10.2165/00002512-200320060-00001. Drugs Aging. 2003. PMID: 12710861 Review. - Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden.
Malm TM, Iivonen H, Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Kanninen K, Salminen A, Auriola S, Van Groen T, Tanila H, Koistinaho J. Malm TM, et al. J Neurosci. 2007 Apr 4;27(14):3712-21. doi: 10.1523/JNEUROSCI.0059-07.2007. J Neurosci. 2007. PMID: 17409235 Free PMC article. - Plasma Aβ as a biomarker for predicting Aβ-PET status in Alzheimer's disease:a systematic review with meta-analysis.
Cheng L, Li W, Chen Y, Lin Y, Wang B, Guo Q, Miao Y. Cheng L, et al. J Neurol Neurosurg Psychiatry. 2022 May;93(5):513-520. doi: 10.1136/jnnp-2021-327864. Epub 2022 Mar 3. J Neurol Neurosurg Psychiatry. 2022. PMID: 35241627 Free PMC article. - Pathological implications of cell cycle re-entry in Alzheimer disease.
Bonda DJ, Lee HP, Kudo W, Zhu X, Smith MA, Lee HG. Bonda DJ, et al. Expert Rev Mol Med. 2010 Jun 29;12:e19. doi: 10.1017/S146239941000150X. Expert Rev Mol Med. 2010. PMID: 20584423 Free PMC article. Review.
References
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–12826. - PubMed
- Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI. Characterization of copper interactions with alzheimer amyloid β peptides: identification of an attomolar-affinity copper binding site on amyloid β1–42. J Neurochem. 2000;75:1219–1233. - PubMed
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β-protein toxicity. Cell. 1994;77:817–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical