Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors - PubMed (original) (raw)
Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors
F Crestani et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The heterogeneity of gamma-aminobutyric acid type A (GABA(A)) receptors contributes to the diversity of neuronal inhibition in the regulation of information processing. Although most GABA(A) receptors are located synaptically, the small population of alpha5GABA(A) receptors is largely expressed extrasynaptically. To clarify the role of the alpha5GABA(A) receptors in the control of behavior, a histidine-to-arginine point mutation was introduced in position 105 of the murine alpha5 subunit gene, which rendered the alpha5GABA(A) receptors diazepam-insensitive. Apart from an incomplete muscle relaxing effect, neither the sedative, anticonvulsant, nor anxiolytic-like activity of diazepam was impaired in alpha5(H105R) mice. However, in hippocampal pyramidal cells, the point mutation resulted in a selective reduction of alpha5GABA(A) receptors, which altered the drug-independent behavior. In line with the role of the hippocampus in certain forms of associative learning, trace fear conditioning, but not delay conditioning or contextual conditioning, was facilitated in the mutant mice. Trace fear conditioning differs from delay conditioning in that the conditioned and unconditioned stimulus are separated by a time interval. Thus, the largely extrasynaptic alpha5GABA(A) receptors in hippocampal pyramidal cells are implicated as control elements of the temporal association of threat cues in trace fear conditioning.
Figures
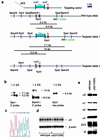
Figure 1
Targeting of the GABAA receptor α5 subunit (GABRA5) gene and molecular analysis. (a) Scheme of targeting strategy. The 3′ probe used for Southern blot analysis is depicted as green box. The “H” and the “R” on the red boxes depicting exon 4 specify whether the exon contains a codon for the naturally occurring histidine or the mutant arginine, respectively, at the amino acid position 105. The neomycin-resistance marker (“Neo”) is flanked by two parallel loxP sites. (b) Southern blot analysis of wild-type embryonic stem cells and mutant clones 1 and 2. (c) Verification of the α5(H105R) point mutation in a homozygous mutant α5(H105R) mouse by DNA sequencing. The codons for amino acids 104–106 are TTC CGG AAC. Exon 4 sequences were amplified by PCR and sequenced on an ABI Prism 310 Genetic Analyzer. (d) Northern blot analysis of the α5 transcript. Total RNA was prepared from whole brain without cerebellum, subjected to agarose gel electrophoresis and blotted onto Nylon membranes (Amersham Pharmacia Hyond N+). The membrane was first hybridized with an α5-exon 4 probe. After etching of the signals, the same blot was hybridized to a β-actin probe to assess the amount of material loaded per lane. (e) Western blot of GABAA receptor subunits. Crude membranes prepared from 3 distinct pools of 10 brains each of wild-type and α5(H105H) mice after removal of the cerebellum were used for Western blotting. To avoid saturation of the signals on the films, Western blotting was performed at protein concentrations ranging between 5 and 40 μg and at least 3 different times of exposure to the x-ray films (ranging from 30 s to 3 min). Densitometric analysis of α5 subunit signals was performed on films derived from blots containing 5, 10, 15, and 20 μg of protein and 3 different exposure times to control for linearity of the signals.
Figure 2
Distribution of diazepam-insensitive sites and induction of LTP. (a) Autoradiographic distribution of benzodiazepine binding sites in parasagital brain sections of wild-type and α5(H105R) mice. (Left) Labeling of both diazepam-sensitive and -insensitive GABAA receptors by incubation with 20 nM [3H]Ro 15–4513. (Right) Incubation with the radioligand in the presence of 10 μM diazepam (labeling of diazepam-insensitive receptors). Nonspecific binding was assessed in the presence of 10 μM flumazenil. (b) Schaffer collateral LTP in wild-type (●) and α5(H105R) mice (▵). After 10 min of baseline recording in hippocampal slices, 20 pulses were given at 100 Hz repeated four times with a 20-s interval for a total of 80 stimuli. The slope (20–80%) of the field excitatory postsynaptic potential was measured and normalized to baseline. The results are given as mean ± SE as percentage of baseline. There was no significant difference in the potentiation throughout the time course of the experiment.
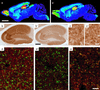
Figure 3
Regional and cellular expression of the α5-subunit protein. Selective loss of extrasynaptic α5GABAA receptors in α5(H105R) mice [a, b, c, and d: wild type; _a_′, _b_′, _c_′, _d_′, and e: α5(H105R) mutant]. (a and _a_′) False-color images depicting the regional distribution of the α5-subunit in parasagital sections of adult mice, as detected by immunoperoxidase staining. Staining in wild type corresponded to that described (11, 31). Note the reduction of staining selectively in the hippocampal formation of mutant mice. (b and _b_′) Enlargement of the hippocampal formation showing the global reduction of α5-subunit immunoreactivity in CA1 and CA3 in the mutant compared with control. (c and _c_′) By comparison, no change in α5-subunit staining intensity was observed in olfactory bulb granule cells (see Table 1 for quantification). (d and _d_′) Images from confocal laser scanning microscopy, depicting a double-immunofluorescence staining for the α5-subunit (red) and gephyrin (green) in the stratum radiatum of CA1. Gephyrin is a postsynaptic marker of GABAergic synapses. The lack of colocalization with the α5-subunit staining reflects the extrasynaptic distribution of α5GABAA receptors. The staining intensity of these receptors is markedly reduced in mutant mice, whereas gephyrin immunoreactivity is unchanged. (e) For comparison, double staining for the α2 subunit (red) and gephyrin (green) reveals the extensive postsynaptic localization of α2GABAA receptors (yellow dots) in α5 mutant mice. (Scale bars: a, _a_′, 2.5 mm; b, _b_′, 0.5 mm; c, _c_′, 25 μm; d, _d_′, and e, 10 μm.)
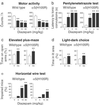
Figure 4
Behavioral responses to diazepam. (a) Motor activity. Diazepam (3–30 mg/kg per os) dose-dependently reduced the activity counts to the same extent in wild-type and α5(H105R) mice [_F_3, 95 = 39.26; **, P < 0.001; n = 9–24]. The two vehicle-treated groups were likewise comparable. (b) Pentylenetetrazole test. Increasing doses of diazepam (1–10 mg/kg per os) protected both wild-type and α5(H105R) mice against pentylenetetrazole-induced convulsions as shown by the similarly increasing delay of occurrence of the tonic convulsion [_F_3,74 = 90.81; **, P < 0.001; n = 6–15]. (c) Elevated plus-maze. Wild-type and α5(H105R) mice displayed a similar increase in the percentage of time spent on the open arms in response to diazepam (1 mg/kg per os) [_F_1,32 = 9.24; **, P < 0.01; n = 9 per group]. The time spent on the enclosed arms did not differ between the two groups. (d) Light–dark choice test. Diazepam (1 mg/kg per os) increased the percentage of time spent in the illuminated area in the same way in wild-type and α5(H105R) mice [_F_1,37 = 17.67; **, P < 0.001; n = 9–12]. The time spent in the dark area did not differ between the two groups. (e) Horizontal wire test. In α5(H105R) mice, diazepam (10 mg/kg per os) failed to produce an impairment of the grasping reflex compared with wild-type mice [_F_3,132 = 5.43; **, P < 0.01; n = 16–24] with a clear genotypic distinction being retained at a higher dose (30 mg/kg). (P < 0.01 versus wild type, Newman–Keuls). Results are expressed as means ± SE. V, vehicle; Dz, diazepam.
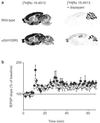
Figure 5
Fear conditioning. (a) Trace fear conditioning. In three learning trials the conditioned stimulus (tone) was followed 1 s later by a foot shock. When tested 48 h later, the α5(H105R) mice showed a higher amount of freezing than wild-type mice over the period of exposure to the tone (solid bar) [_F_1,84 = 4.44, *, P < 0.05; n = 7 per group]. No difference in the freezing response was observed during the first 3 min of exposure to a modified context. (b) Delay fear conditioning. In three learning trials, the tone coterminated with the foot shock. When tested 48 h later, the mean percentage of time spent freezing to the tone (8 min) was similar in wild-type and α5(H105R) mice (n = 8 per group). No group difference was seen in the freezing response to a modified context (first 3 min). (c) Contextual fear conditioning. After three trials of exposure to a foot shock, wild-type and α5(H105R) mice displayed a similar mean percentage of time freezing when re-exposed to the same context 24 h later (n = 8 per group). Results are expressed as means ± SE.
Similar articles
- GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear.
Yee BK, Hauser J, Dolgov VV, Keist R, Möhler H, Rudolph U, Feldon J. Yee BK, et al. Eur J Neurosci. 2004 Oct;20(7):1928-36. doi: 10.1111/j.1460-9568.2004.03642.x. Eur J Neurosci. 2004. PMID: 15380015 - Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice.
Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Misane I, et al. Hippocampus. 2005;15(4):418-26. doi: 10.1002/hipo.20067. Hippocampus. 2005. PMID: 15669102 - Alpha5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons.
Bonin RP, Martin LJ, MacDonald JF, Orser BA. Bonin RP, et al. J Neurophysiol. 2007 Oct;98(4):2244-54. doi: 10.1152/jn.00482.2007. Epub 2007 Aug 22. J Neurophysiol. 2007. PMID: 17715197 - The sedative but not the memory-blocking properties of ethanol are modulated by α5-subunit-containing γ-aminobutyric acid type A receptors.
Martin LJ, Zurek AA, Bonin RP, Oh GH, Kim JH, Mount HT, Orser BA. Martin LJ, et al. Behav Brain Res. 2011 Mar 1;217(2):379-85. doi: 10.1016/j.bbr.2010.11.008. Epub 2010 Nov 9. Behav Brain Res. 2011. PMID: 21070817 - Hippocampal activity, but not plasticity, is required for early consolidation of fear conditioning with a short trace interval.
Burman MA, Gewirtz JC. Burman MA, et al. Eur J Neurosci. 2007 Apr;25(8):2483-90. doi: 10.1111/j.1460-9568.2007.05493.x. Eur J Neurosci. 2007. PMID: 17445243
Cited by
- Detoxification Improves Multidomain Cognitive Dysfunction in High-Dose Benzodiazepine Abusers.
Federico A, Lugoboni F, Mantovani E, Martini A, Morbioli L, Casari R, Faccini M, Tamburin S. Federico A, et al. Front Neurosci. 2020 Jul 21;14:747. doi: 10.3389/fnins.2020.00747. eCollection 2020. Front Neurosci. 2020. PMID: 32848544 Free PMC article. - Analysis of β-Subunit-dependent GABAA Receptor Modulation and Behavioral Effects of Valerenic Acid Derivatives.
Khom S, Hintersteiner J, Luger D, Haider M, Pototschnig G, Mihovilovic MD, Schwarzer C, Hering S. Khom S, et al. J Pharmacol Exp Ther. 2016 Jun;357(3):580-90. doi: 10.1124/jpet.116.232983. Epub 2016 Apr 18. J Pharmacol Exp Ther. 2016. PMID: 27190170 Free PMC article. - Extrasynaptic localization is essential for α5GABAA receptor modulation of dopamine system function.
McCoy AM, Prevot TD, Mian MY, Sharmin D, Ahmad AN, Cook JM, Sibille EL, Lodge DJ. McCoy AM, et al. eNeuro. 2024 Feb 27;11(3):ENEURO.0344-23.2023. doi: 10.1523/ENEURO.0344-23.2023. Online ahead of print. eNeuro. 2024. PMID: 38413199 Free PMC article. - Stress, ethanol, and neuroactive steroids.
Biggio G, Concas A, Follesa P, Sanna E, Serra M. Biggio G, et al. Pharmacol Ther. 2007 Oct;116(1):140-71. doi: 10.1016/j.pharmthera.2007.04.005. Epub 2007 May 8. Pharmacol Ther. 2007. PMID: 17555824 Free PMC article. Review. - GABAA Receptors Are Well Preserved in the Hippocampus of Aged Mice.
Palpagama TH, Sagniez M, Kim S, Waldvogel HJ, Faull RL, Kwakowsky A. Palpagama TH, et al. eNeuro. 2019 Aug 14;6(4):ENEURO.0496-18.2019. doi: 10.1523/ENEURO.0496-18.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31340951 Free PMC article.
References
- Cobb S R, Buhl E H, Halasy K, Paulsen O, Somogyi P. Nature (London) 1995;378:75–78. - PubMed
- Whittington M A, Traub R D, Jeffreys J G. Nature (London) 1995;373:612–615. - PubMed
- Gupta A, Wang Y, Markram H. Science. 2000;287:273–278. - PubMed
- Tamas G, Buhl E H, Lorincz A, Somogyi P. Nat Neurosci. 2000;3:366–371. - PubMed
- Wallenstein G V, Eichenbaum H, Hasselmo E. Trends Neuosci. 1998;21:317–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials