Gene targeting of Gemin2 in mice reveals a correlation between defects in the biogenesis of U snRNPs and motoneuron cell death - PubMed (original) (raw)
Gene targeting of Gemin2 in mice reveals a correlation between defects in the biogenesis of U snRNPs and motoneuron cell death
Sibylle Jablonka et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Neuronal degeneration in spinal muscular atrophy is caused by reduced expression of the survival motor neuron (SMN) protein. SMN and the tightly interacting Gemin2 form part of a macromolecular complex (SMN complex) that mediates assembly of spliceosomal small nuclear ribonucleoproteins (U snRNPs). We used mouse genetics to investigate the function of this complex in motoneuron maintenance. Reduced Smn/Gemin2 protein levels lead to disturbed U snRNP assembly as indicated by reduced nuclear accumulation of Sm proteins. This finding correlates with enhanced motoneuron degeneration in Gemin2(+/-)/Smn(+/-) mice. Our data provide in vivo evidence that impaired production of U snRNPs contributes to motoneuron degeneration.
Figures
Figure 1
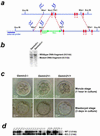
Homologous recombination of the Gemin2 locus in mice and characterization of wild-type and Gemin2 targeted preimplantation embryos. (a) An _EBFP_-_Neo_-cassette (large green arrows) was inserted into exon 1 of the Gemin2 gene by homologous recombination (dashed lines in red) in mouse embryonic stem cells. Small blue arrows indicate the position of primers used for genotyping and the horizontal blue bar indicates the DNA probe used for Southern blot analysis of _Eco_RI-digested genomic DNA. Exons are marked in red. (b) Southern blot analysis of Gemin2+/− and wild-type mice with _Eco_RI-digested genomic DNA. All Gemin2+/− clones identified by PCR showed an _Eco_RI fragment of 6.5 kb indicative for the mutated allele along with the 9.0-kb wild-type allele fragment. (c) No morphological alterations were observed at the morula (Upper) and blastocyst stages (Lower) in _Gemin2_−/−, Gemin2+/−, and wild-type embryos. (d) Genotyping by PCR and subsequent Southern blotting showed products corresponding to the wild type (WT, 1.6 kb) and the mutant allele (KO, 1.2 kb).
Figure 2
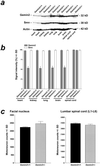
Quantification of Gemin2 and Smn protein levels in tissues of 7-month-old heterozygous and wild-type Gemin2 mice. (a) Gemin2 protein was detected by Western blotting using a Gemin2-specific antiserum (Top). The signal for Gemin2 was reduced in heart, lung, kidney, and brain of Gemin2+/− mice. In contrast, Gemin2 protein levels were unchanged in spinal cord of Gemin2+/− mice. Western blotting of the same extract with monoclonal anti-Smn antibody (Middle) revealed similar signal intensities in tissues of wild-type and Gemin2+/− mice. (Bottom) Reprobing of the Western blot with a monoclonal anti-actin antibody as a control for equal loading of the gel. (b) Gemin2 and Smn protein content in various tissues of 7-month-old wild-type and Gemin2+/− mice was determined by scanning and quantification of the intensity of the Gemin2 and Smn immunoreactive bands from Western blots. Gemin2+/− mice showed similar Gemin2 protein levels in spinal cord tissue. Signal intensities for Gemin2 in heart, kidney, lung, and brain of Gemin2+/− mice (gray bars) were significantly reduced of about 50% (*). Smn signal intensity in each tissue was similar in Gemin2+/− and wild-type mice (white bars). (c) Quantification of motoneurons in the facial nucleus (Left) and in the spinal cord (Right) of heterozygous deficient and wild-type Gemin2 mice. Motoneuron counts in the facial nucleus and in the spinal cord of heterozygous deficient and wild-type Gemin2 mice revealed no differences.
Figure 3
Quantification of the Gemin2 and Smn protein levels in spinal cord of 5-month-old Gemin2 and Smn heterozygous deficient mice. Gemin2 and Smn protein levels in spinal cord of wild-type, Smn+/−, Smn+/−/Gemin2+/−, and Gemin2+/− mice were compared by quantification of the intensities of Gemin2 and Smn immunoreactive bands from Western blots using a Gemin2-specific antiserum and a monoclonal anti-Smn antibody. Similar protein expression of Gemin2 and Smn was observed in spinal cord tissue of wild-type and Gemin2+/− mice. Only 50% of Smn (*, white bar) and Gemin2 (*, gray bar) protein was expressed in spinal cord of Smn+/− mice. Immunodetection of Gemin2 in spinal cord of Smn+/−/Gemin2+/− mice showed a stronger reduction (−75%) compared with Smn (−50%) (P < 0.05).
Figure 4
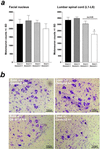
Quantitative analysis of facial and lumbar spinal motoneurons of 5-month-old Gemin2+/−, Smn+/−, Gemin2+/−/Smn+/−, and wild-type mice. (a) Counting of facial motoneurons in 5-month-old Smn+/−, Gemin2+/−, Smn+/−/Gemin2+/−, and wild-type littermates revealed no differences (Left). In contrast, the number of lumbar spinal motoneurons in Smn+/−/Gemin2+/− mice (*) was significantly reduced (−34%) in comparison to wild-type, Gemin2+/−, and Smn+/− mice (P < 0.05; Right). (b) Nissl staining of lumbar spinal motoneurons in Gemin2+/−, Smn+/−, Smn+/−/Gemin2+/−, and wild-type mice.
Figure 5
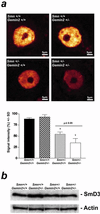
Sm protein immunoreactivity in the nucleus of spinal motoneurons of 5-month-old Gemin2+/−, Smn+/−, Gemin2+/−/Smn+/−, and wild-type mice. (a) The monoclonal anti-Sm protein antibody Y12 was used for immunohistochemical analysis of the Sm protein distribution in the nucleus of spinal motoneurons of 5-month-old Gemin2+/−, Smn+/−, Gemin2+/−/Smn+/−, and wild-type mice. A significant reduction of Sm proteins in the nucleus of spinal motoneurons was detectable in Smn/Gemin2 double heterozygous deficient mice (*) in comparison to wild type, Gemin2+/−, and Smn+/− motoneurons. Distribution of the Sm proteins within the cell body of each genotype was not altered. (Lower) The semiquantitative analysis of Sm protein levels in spinal motoneuron nuclei. Reduced Sm protein signal intensity was measured in spinal motoneurons of Smn+/− (−39%, *) and even more (P < 0.05) in Smn+/−/Gemin2+/− mice in comparison to wild-type and Gemin2+/− mice. (b) Similar signal intensities for SmD3 proteins in spinal cord of wild-type, _Gemin_2+/−, Smn+/−, and Smn/Gemin2 double heterozygous-deficient mice were observed. The anti-actin antibody was used as a control showing equal protein concentration in each lane.
Similar articles
- The survival of motor neurons protein determines the capacity for snRNP assembly: biochemical deficiency in spinal muscular atrophy.
Wan L, Battle DJ, Yong J, Gubitz AK, Kolb SJ, Wang J, Dreyfuss G. Wan L, et al. Mol Cell Biol. 2005 Jul;25(13):5543-51. doi: 10.1128/MCB.25.13.5543-5551.2005. Mol Cell Biol. 2005. PMID: 15964810 Free PMC article. - The SMN binding protein Gemin2 is not involved in motor axon outgrowth.
McWhorter ML, Boon KL, Horan ES, Burghes AH, Beattie CE. McWhorter ML, et al. Dev Neurobiol. 2008 Feb 1;68(2):182-94. doi: 10.1002/dneu.20582. Dev Neurobiol. 2008. PMID: 18000835 - Reduced U snRNP assembly causes motor axon degeneration in an animal model for spinal muscular atrophy.
Winkler C, Eggert C, Gradl D, Meister G, Giegerich M, Wedlich D, Laggerbauer B, Fischer U. Winkler C, et al. Genes Dev. 2005 Oct 1;19(19):2320-30. doi: 10.1101/gad.342005. Genes Dev. 2005. PMID: 16204184 Free PMC article. - The SMN complex.
Gubitz AK, Feng W, Dreyfuss G. Gubitz AK, et al. Exp Cell Res. 2004 May 15;296(1):51-6. doi: 10.1016/j.yexcr.2004.03.022. Exp Cell Res. 2004. PMID: 15120993 Review. - Fishing for a mechanism: using zebrafish to understand spinal muscular atrophy.
Beattie CE, Carrel TL, McWhorter ML. Beattie CE, et al. J Child Neurol. 2007 Aug;22(8):995-1003. doi: 10.1177/0883073807305671. J Child Neurol. 2007. PMID: 17761655 Review.
Cited by
- Motor neuron biology and disease: A current perspective on infantile-onset spinal muscular atrophy.
Jha NN, Kim JK, Monani UR. Jha NN, et al. Future Neurol. 2018 Aug;13(3):161-172. doi: 10.2217/fnl-2018-0008. Epub 2018 Jul 6. Future Neurol. 2018. PMID: 31396020 Free PMC article. - Negative cooperativity between Gemin2 and RNA provides insights into RNA selection and the SMN complex's release in snRNP assembly.
Yi H, Mu L, Shen C, Kong X, Wang Y, Hou Y, Zhang R. Yi H, et al. Nucleic Acids Res. 2020 Jan 24;48(2):895-911. doi: 10.1093/nar/gkz1135. Nucleic Acids Res. 2020. PMID: 31799625 Free PMC article. - Role of survival motor neuron complex components in small nuclear ribonucleoprotein assembly.
Ogawa C, Usui K, Ito F, Itoh M, Hayashizaki Y, Suzuki H. Ogawa C, et al. J Biol Chem. 2009 May 22;284(21):14609-17. doi: 10.1074/jbc.M809031200. Epub 2009 Mar 25. J Biol Chem. 2009. PMID: 19321448 Free PMC article. - Gemin4 is an essential gene in mice, and its overexpression in human cells causes relocalization of the SMN complex to the nucleoplasm.
Meier ID, Walker MP, Matera AG. Meier ID, et al. Biol Open. 2018 Feb 1;7(2):bio032409. doi: 10.1242/bio.032409. Biol Open. 2018. PMID: 29371219 Free PMC article. - Spinal Muscular Atrophy: From Defective Chaperoning of snRNP Assembly to Neuromuscular Dysfunction.
Lanfranco M, Vassallo N, Cauchi RJ. Lanfranco M, et al. Front Mol Biosci. 2017 Jun 8;4:41. doi: 10.3389/fmolb.2017.00041. eCollection 2017. Front Mol Biosci. 2017. PMID: 28642865 Free PMC article. Review.
References
- Crawford T O. Neurology. 1996;46:335–340. - PubMed
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, et al. Cell. 1995;80:155–165. - PubMed
- Endrizzi M, Huang S, Scharf J M, Kelter A R, Wirth B, Kunkel L M, Miller W, Dietrich W F. Genomics. 1999;60:137–151. - PubMed
- Wirth B. Hum Mutat. 2000;15:228–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases