The hippocampus and disambiguation of overlapping sequences - PubMed (original) (raw)
The hippocampus and disambiguation of overlapping sequences
Kara L Agster et al. J Neurosci. 2002.
Abstract
Recent models of hippocampal function emphasize its potential role in disambiguating sequences of events that compose distinct episodic memories. In this study, rats were trained to distinguish two overlapping sequences of odor choices. The capacity to disambiguate the sequences was measured by the critical odor choice after the overlapping elements of the sequences. When the sequences were presented in rapid alternation, damage to the hippocampus, produced either by infusions of the neurotoxin ibotenic acid or by radiofrequency current, produced a severe deficit, although animals with radiofrequency lesions relearned the task. When the sequences were presented spaced apart and in random order, animals with radiofrequency hippocampal lesions could perform the task. However, they failed when a memory delay was imposed before the critical choice. These findings support the hypothesis that the hippocampus is involved in representing sequences of nonspatial events, particularly when interference between the sequences is high or when animals must remember across a substantial delay preceding items in a current sequence.
Figures
Fig. 1.
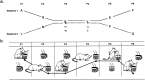
The odor sequence task. a, The two odor sequences are indicated by letters. In performing each sequence, the rat selected between vertically aligned odors in each sequence; in both sequences, X was to be selected over_W_, and Y over Z. b, Illustration of an example trial on sequence 1. The location where the odors are presented is randomly determined. On the first four pairs (P1–P4) and pair 6 (P6), the animal was required to lift the perforated lid and dig from the cup containing the reward; the lid of the alternative choices was “locked.” On pair 5 (P5), no lids are used, and the first choice is scored. +, Reinforced odor.
Fig. 2.
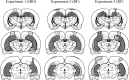
Reconstructions of the smallest and largest hippocampal lesions. Coronal sections are adapted from Swanson (1992).Light gray refers to the largest volume of hippocampus damaged; dark gray indicates smallest lesion.
Fig. 3.
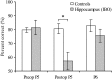
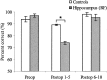
Performance (mean ± SE) of control rats and rats with ibotenic acid (IBO) hippocampal lesions on pair 5 (P5) and pair 6 (P6) tests. *p < 0.025.
Fig. 4.
Performance (mean ± SE) on pair 5 judgements by control rats and rats with ibotenic acid (IBO) hippocampal lesions on the first and second five-session blocks of postoperative sessions. *p < 0.025.
Fig. 5.
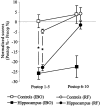
Performance (mean ± SE) of control rats and rats with radiofrequency (RF) hippocampal lesions on preoperative and postoperative pair 5 testing. The postoperative performance is divided into two blocks of five sessions. *p < 0.025.
Fig. 6.
Performance (mean ± SE) of control rats and rats with ibotenic acid (IBO) or radiofrequency (RF) hippocampal lesions on postoperative pair 5 choices. Data are shown in two blocks of five sessions. The normalized scores were obtained by subtracting the preoperative performance level from the performance on each postoperative block. Note that a negative score indicates a decrease in performance from the preoperative level. *p < 0.025.
Fig. 7.
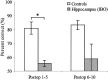
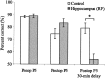
Performance (mean ± SE) of control rats and rats with radiofrequency hippocampal lesions on preoperative and postoperative pair 5 choices in the random-presentation version of the sequence disambiguation task. Scores are shown for the final stage of preoperative training and in postoperative testing with minimal and 30 min delay before presentation of pair 5. *p < 0.025.
Similar articles
- Combined lesions of hippocampus and subiculum Do not produce deficits in a nonspatial social olfactory memory task.
Burton S, Murphy D, Qureshi U, Sutton P, O'Keefe J. Burton S, et al. J Neurosci. 2000 Jul 15;20(14):5468-75. doi: 10.1523/JNEUROSCI.20-14-05468.2000. J Neurosci. 2000. PMID: 10884330 Free PMC article. - Hippocampal neurons encode different episodes in an overlapping sequence of odors task.
Ginther MR, Walsh DF, Ramus SJ. Ginther MR, et al. J Neurosci. 2011 Feb 16;31(7):2706-11. doi: 10.1523/JNEUROSCI.3413-10.2011. J Neurosci. 2011. PMID: 21325539 Free PMC article. - Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation.
Dudchenko PA, Wood ER, Eichenbaum H. Dudchenko PA, et al. J Neurosci. 2000 Apr 15;20(8):2964-77. doi: 10.1523/JNEUROSCI.20-08-02964.2000. J Neurosci. 2000. PMID: 10751449 Free PMC article. - On the role of the hippocampus in learning and memory in the rat.
Jarrard LE. Jarrard LE. Behav Neural Biol. 1993 Jul;60(1):9-26. doi: 10.1016/0163-1047(93)90664-4. Behav Neural Biol. 1993. PMID: 8216164 Review. - Complementary roles of hippocampus and medial entorhinal cortex in episodic memory.
Lipton PA, Eichenbaum H. Lipton PA, et al. Neural Plast. 2008;2008:258467. doi: 10.1155/2008/258467. Neural Plast. 2008. PMID: 18615199 Free PMC article. Review.
Cited by
- Cognitive mechanisms of memory for order in rhesus monkeys (Macaca mulatta).
Templer VL, Hampton RR. Templer VL, et al. Hippocampus. 2013 Mar;23(3):193-201. doi: 10.1002/hipo.22082. Epub 2012 Nov 29. Hippocampus. 2013. PMID: 23197396 Free PMC article. - Contributions of medial temporal lobe and striatal memory systems to learning and retrieving overlapping spatial memories.
Brown TI, Stern CE. Brown TI, et al. Cereb Cortex. 2014 Jul;24(7):1906-22. doi: 10.1093/cercor/bht041. Epub 2013 Feb 28. Cereb Cortex. 2014. PMID: 23448868 Free PMC article. - The neural basis of nonvisual object recognition memory in the rat.
Albasser MM, Olarte-Sánchez CM, Amin E, Horne MR, Newton MJ, Warburton EC, Aggleton JP. Albasser MM, et al. Behav Neurosci. 2013 Feb;127(1):70-85. doi: 10.1037/a0031216. Epub 2012 Dec 17. Behav Neurosci. 2013. PMID: 23244291 Free PMC article. - The role of hippocampal glutamate receptor-A-dependent synaptic plasticity in conditional learning: the importance of spatiotemporal discontiguity.
Schmitt WB, Arianpour R, Deacon RM, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM. Schmitt WB, et al. J Neurosci. 2004 Aug 18;24(33):7277-82. doi: 10.1523/JNEUROSCI.1093-04.2004. J Neurosci. 2004. PMID: 15317854 Free PMC article. - Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events.
Farovik A, Dupont LM, Eichenbaum H. Farovik A, et al. Learn Mem. 2009 Dec 22;17(1):12-17. doi: 10.1101/lm.1616209. Print 2010 Jan. Learn Mem. 2009. PMID: 20028733 Free PMC article.
References
- Anagnostaras SG, Gale GD, Fanselow MS (2002) The hippocampus and Pavlovian fear conditioning: reply to Bast et al. Hippocampus, in press. - PubMed
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. - PubMed
- Chiba AA, Kesner RP, Reynolds AM. Memory for spatial location as a function of temporal lag in rats: role of hippocampus and medial prefrontal cortex. Behav Neural Biol. 1994;61:123–131. - PubMed
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical