IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype - PubMed (original) (raw)
Comparative Study
IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype
P'ng Loke et al. BMC Immunol. 2002.
Abstract
Background: "Alternatively-activated" macrophages are found in Th2-mediated inflammatory settings such as nematode infection and allergic pulmonary inflammation. Due in part to a lack of markers, these cells have not been well characterized in vivo and their function remains unknown.
Results: We have used murine macrophages elicited by nematode infection (NeM(phi)) as a source of in vivo derived alternatively activated macrophages. Using three distinct yet complementary molecular approaches we have established a gene expression profile of alternatively activated macrophages and identified macrophage genes that are regulated in vivo by IL-4. First, genes abundantly expressed were identified by an expressed sequence tag strategy. Second, an array of 1176 known mouse genes was screened for differential expression between NeM(phi) from wild type or IL-4 deficient mice. Third, a subtractive library was screened to identify novel IL-4 dependent macrophage genes. Differential expression was confirmed by real time RT-PCR analysis.
Conclusions: Our data demonstrate that alternatively activated macrophages generated in vivo have a gene expression profile distinct from any macrophage population described to date. Several of the genes we identified, including those most abundantly expressed, have not previously been associated with macrophages and thus this study provides unique new information regarding the phenotype of macrophages found in Th2-mediated, chronic inflammatory settings. Our data also provide additional in vivo evidence for parallels between the inflammatory processes involved in nematode infection and allergy.
Figures
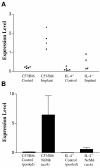
Figure 1
Arginase 1 expression is increased in response to parasite implant in C57BL/6 but not IL-4 -/- mice. Real-time PCR analysis of arginase 1 expression by total cells (A) or F4/80 purified macrophages (B) obtained from peritoneal lavages of implanted or control mice. For the purified NeMφ, each cDNA sample was obtained from individual mice. For the purified control macrophages, the cDNA samples represent pooled macrophages from 5 mice. Expression levels were measured as a percentage of β-Actin expression. Error bars represent one SD from the mean of each analysis.
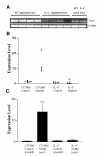
Figure 2
Mouse array analysis and real time PCR verfication. (A) Chart of the genes that showed the highest differential expression between NeMφ and thioglycollate-recruited macrophages. Values represent the difference between the gene expression in WT NeMφ and thioglycollate-recruited Mφ (arbitrary units). (B) Chart showing the difference between the gene expression in WT NeMφ and IL-4 -/- NeMφ. Genes selected for real-time PCR analysis are marked with an asterisk (*). (C) An example of a section of the Atlas array that contains MIP-1α (c), MIP-1β (d), MIP-2 (e). The left panel is probed with WT NeMφ and the right panel with IL-4-/- NeMφ. This section also contains the genes: prothymosin beta 4 (a), insulin-like growth factor IA (b), GADPH (f), myosin I alpha (g), and ornithine decarboxylase (h), which are all not differentially expressed. (D) Verification of Atlas array results by real-time PCR. cDNA samples from F4/80 purified macrophages from either resident peritoneal cells (con) or from parasite implanted mice (NeMφ) were diluted and normalized to contain equal levels of β-actin transcript (data not shown) before quantification of the different genes. Expression levels are shown in arbitrary units that are based on a comparison with β-actin expression (designated as 10,000 units). The results shown represent the mean values of 2 experiments with independent sources of experimental macrophage RNA.
Figure 3
FIZZ1/RELMα expression is increased in response to parasite implant in C57BL/6 but not IL-4 -/- mice. Semiquantitative RT-PCR of FIZZ1/RELMα and β-actin was performed on total RNA samples from the peritoneal lavages of individual mice (A). Real Time PCR analysis of FIZZ1 expression by total cells (B) or F4/80 purified macrophages (C) obtained from peritoneal lavages of implanted or naïve control mice. For the purified NeMφ, each cDNA sample was obtained from individual mice. For the purified control macrophages, the cDNA samples represent pooled macrophages from groups of 2 or 3 mice for a total of 5 mice per control group. Expression levels were measured as a percentage of β-actin expression. Error bars represent one SD from the mean.
Similar articles
- Induction of IL-4Rα-dependent microRNAs identifies PI3K/Akt signaling as essential for IL-4-driven murine macrophage proliferation in vivo.
Rückerl D, Jenkins SJ, Laqtom NN, Gallagher IJ, Sutherland TE, Duncan S, Buck AH, Allen JE. Rückerl D, et al. Blood. 2012 Sep 13;120(11):2307-16. doi: 10.1182/blood-2012-02-408252. Epub 2012 Aug 1. Blood. 2012. PMID: 22855601 Free PMC article. - Alternative activation of macrophages by filarial nematodes is MyD88-independent.
Mylonas KJ, Hoeve MA, MacDonald AS, Allen JE. Mylonas KJ, et al. Immunobiology. 2013 Apr;218(4):570-8. doi: 10.1016/j.imbio.2012.07.006. Epub 2012 Jul 21. Immunobiology. 2013. PMID: 22884360 Free PMC article. - Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages.
Raes G, De Baetselier P, Noël W, Beschin A, Brombacher F, Hassanzadeh Gh G. Raes G, et al. J Leukoc Biol. 2002 Apr;71(4):597-602. J Leukoc Biol. 2002. PMID: 11927645 - Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease.
Van Dyken SJ, Locksley RM. Van Dyken SJ, et al. Annu Rev Immunol. 2013;31:317-43. doi: 10.1146/annurev-immunol-032712-095906. Epub 2013 Jan 3. Annu Rev Immunol. 2013. PMID: 23298208 Free PMC article. Review.
Cited by
- M-CSF, IL-6, and TGF-β promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery.
Li Z, Xiao J, Xu X, Li W, Zhong R, Qi L, Chen J, Cui G, Wang S, Zheng Y, Qiu Y, Li S, Zhou X, Lu Y, Lyu J, Zhou B, Zhou J, Jing N, Wei B, Hu J, Wang H. Li Z, et al. Sci Adv. 2021 Mar 12;7(11):eabb6260. doi: 10.1126/sciadv.abb6260. Print 2021 Mar. Sci Adv. 2021. PMID: 33712456 Free PMC article. - Chitinase dependent control of protozoan cyst burden in the brain.
Nance JP, Vannella KM, Worth D, David C, Carter D, Noor S, Hubeau C, Fitz L, Lane TE, Wynn TA, Wilson EH. Nance JP, et al. PLoS Pathog. 2012;8(11):e1002990. doi: 10.1371/journal.ppat.1002990. Epub 2012 Nov 29. PLoS Pathog. 2012. PMID: 23209401 Free PMC article. - An efferocytosis-induced, IL-4-dependent macrophage-iNKT cell circuit suppresses sterile inflammation and is defective in murine CGD.
Zeng MY, Pham D, Bagaitkar J, Liu J, Otero K, Shan M, Wynn TA, Brombacher F, Brutkiewicz RR, Kaplan MH, Dinauer MC. Zeng MY, et al. Blood. 2013 Apr 25;121(17):3473-83. doi: 10.1182/blood-2012-10-461913. Epub 2013 Feb 20. Blood. 2013. PMID: 23426944 Free PMC article. - Thymic stromal lymphopoietin is up-regulated in the skin of patients with systemic sclerosis and induces profibrotic genes and intracellular signaling that overlap with those induced by interleukin-13 and transforming growth factor β.
Christmann RB, Mathes A, Affandi AJ, Padilla C, Nazari B, Bujor AM, Stifano G, Lafyatis R. Christmann RB, et al. Arthritis Rheum. 2013 May;65(5):1335-46. doi: 10.1002/art.37859. Arthritis Rheum. 2013. PMID: 23335246 Free PMC article. - Induction of protective immunity against cryptococcosis.
Wozniak KL, Hardison S, Olszewski M, Wormley FL Jr. Wozniak KL, et al. Mycopathologia. 2012 Jun;173(5-6):387-94. doi: 10.1007/s11046-011-9505-8. Epub 2011 Dec 6. Mycopathologia. 2012. PMID: 22143898 Review.
References
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;3:503–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources