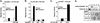A structural model of the constitutive androstane receptor defines novel interactions that mediate ligand-independent activity - PubMed (original) (raw)
A structural model of the constitutive androstane receptor defines novel interactions that mediate ligand-independent activity
Isabelle Dussault et al. Mol Cell Biol. 2002 Aug.
Abstract
Unlike classical nuclear receptors that require ligand for transcriptional activity, the constitutive androstane receptor (CAR) is active in the absence of ligand. To determine the molecular contacts that underlie this constitutive activity, we created a three-dimensional model of CAR and verified critical structural features by mutational analysis. We found that the same motifs that facilitate ligand-dependent activity in classical receptors also mediated constitutive activity in CAR. This raises a critical question: how are these motifs maintained in an active conformation in unliganded CAR? The model identified several novel interactions that account for this activity. First, CAR possesses a short loop between helix 11 and the transactivation domain (helix 12), as well as a short carboxy-terminal helix. Together, these features favor ligand-independent docking of the transactivation domain in a position that is characteristic of ligand-activated receptors. Second, this active conformation is further stabilized by a charge-charge interaction that anchors the carboxy-terminal activation domain to helix 4. Mutational analysis of these interactions provides direct experimental support for this model. We also show that ligand-mediated repression of constitutive activity reflects both a displacement of coactivator and a recruitment of corepressor. Our data demonstrate that CAR utilizes the same conserved structural motifs and coregulator proteins as originally defined for classical nuclear receptors. Despite these remarkable similarities, our model demonstrates how a few critical changes in CAR can dramatically reverse the transcriptional activity of this protein.
Figures
FIG. 1.
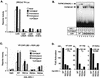
Mouse CAR activity requires the same structural motifs as ligand-dependent activity by other nuclear receptors. (A) CV-1 cells were cotransfected with different CAR expression plasmids, a luciferase reporter construct containing two copies of the βRE2 response element, and CMX-βgal. Cells were treated with ligands (TCPOBOP at 10 μM and androstanol at 5 μM) for 40 h before luciferase and β-galactosidase activities were measured. (B) Coactivator recruitment assay was performed by mixing wild-type (WT) or mutant CAR, RXR, a 32P-labeled βRE probe, and the bacterially expressed RIDs of SRC-1. TCPOBOP (10 μM) was added to the mix where indicated. Heterodimer formation and migration were not changed by the various receptor mutants. (C) A mammalian two-hybrid experiment was performed by transfecting CV-1 cells with expression plasmids for Gal-SRC-1, VP-CAR LBD, the RXR LBD, and a luciferase reporter containing four copies of a Gal4 response element (UASGx4). Cells were treated for 40 h with ligands before luciferase and β-galactosidase activities were measured. Gal-SRC-1 refers to a fusion between the Gal4 DNA-binding domain and the three RIDs of SRC-1 (wild-type RIDs [WT] or mutations in either of the three LXXLL motifs [RID1m, RID2m, and RID3m]). Western blot analysis indicated that all mutants were expressed to levels equivalent to those of the wild-type proteins (data not shown). (D) Same as panel C except that VP-VDR LBD, VP-TRβ LBD, or VP-RARα LBD was used and the cells were treated with the corresponding ligands, as indicated. WT, wild type. Reporter activity refers to the luciferase value divided by the β-galactosidase value for each point. Fold activation represents the reporter activity of the receptor in the presence of ligand divided by the reporter activity of the same receptor in the absence of ligand.
FIG. 2.
Mouse CAR requires the RXR LBD, but not its coactivator interaction domains, for full activity. (A) A mammalian two-hybrid assay was performed as described for Fig. 1C except that no ligands were added. (B) A mammalian two-hybrid assay was performed as for Fig. 1C. (C) CV-1 cells were cotransfected with the indicated RXR expression plasmids, a luciferase reporter construct containing three copies of an RXRE, and CMX-βgal. Cells were treated for 40 h with 100 nM LG268 before luciferase and β-galactosidase activities were measured. (D) The coactivator recruitment assay was performed by mixing wild-type (WT) or mutant RXR, a 32P-labeled DR-1 probe, and the bacterially expressed RIDs of SRC-1. LG268 (100 nM) was added to the mix where indicated. Only the bound DNA complexes are shown. Heterodimer formation and migration were not changed by the various receptor mutants. (E) A mammalian two-hybrid experiment was performed by transfecting CV-1 cells with expression vectors for Gal-SRC-1, VP-CAR LBD, the indicated RXR LBD mutants, and a luciferase reporter containing four copies of a Gal4 response element (UASGx4). No ligands were added. (F) Coactivator recruitment assay was performed by mixing CAR, wild-type or mutant RXR, a 32P-labeled βRE probe, and the bacterially expressed RIDs of SRC-1. Only the bound DNA complexes are shown.
FIG. 3.
Repression of CAR is mediated by an interaction with SMRT. (A) A mammalian two-hybrid assay was performed by transfecting CV-1 cells with expression plasmids for Gal-SMRT, VP-CAR LBD, the RXR LBD, and the UASGx4 luciferase reporter. Cells were treated for 40 h with ligands before luciferase and β-galactosidase activities were measured. Gal-SMRT refers to a fusion between the Gal4 DNA-binding domain and the two RIDs of SMRT. (B) A mammalian two-hybrid assay was performed as for panel A except that the Gal-SMRT constructs contained either the isolated RID1 or RID2. Western blot analysis indicated that all mutants were expressed to levels equivalent to those of the wild-type (WT) proteins (data not shown). (C) A mammalian two-hybrid assay was performed as for panel A with wild-type CAR or with a mutant lacking H12/AF2 (ΔAF2). (D) A coactivator recruitment assay was performed by mixing the indicated CAR and RXR constructs, a 32P-labeled βRE2 probe, and the bacterially expressed SMRT RIDs. Heterodimer formation and migration were not changed by the various receptor mutants.
FIG. 4.
Homology model of the CAR ligand-binding domain. (A) Structure-based amino acid sequence alignment of human PXR and mouse CAR ligand-binding domains. Cylinders represent α-helices in PXR as defined in Protein Data Bank file 1ILH. Arrows represent β-strands. Specific α-helices (H) and β-strands (β) are identified by number. PXR lacks H2 and H6, which are found in most other LBDs. Identical residues are shown on a black background. Homologous residues are shaded gray. Asterisks indicate residues that line the internal ligand-binding cavity in the crystal structure of PXR or the model of CAR. Helices 10 and 11 are indicated as one continuous helix, as described in the PXR crystal structure (51). (B) Homology model of mouse CAR based on the crystal structure of human PXR. Specific structural elements are labeled. TCPOBOP (white) is shown in one of several possible conformations within the ligand-binding cavity, as described in Materials and Methods. Androstanol (blue) can mimic the hydrophobic side chains of H12 (L352, L353, I356, and C357). Thus, androstanol is shown superimposed on H12/AF2 and docked in the groove normally occupied by this helix when it assumes the active conformation (see Discussion). In this model, the residues that line the androstanol pocket are N175 (backbone oxygen only), T176, V179, Q180, K205, A208, V209, S337, Y338, L340, Q341, M349, and T350. (C) Top view of the mouse CAR homology model. Pink, solid rendering of mouse CAR LBD residues 116 to 344. Blue, ribbon rendering of residues 345 to 358, which constitute H12/AF2 and the loop that precedes it. L353, I356, and C357, residues that mimic the three leucines in the canonical LXXLL motif of CAR, are shown in blue space-filling representation. Cyan, L352, the residue that precedes the LXXLL motif and forms part of the hydrophobic cleft in which coactivator binds. Red, ribbon rendering of SRC-1 coactivator peptide. The three leucines of the SRC-1 RID2 LXXLL motif (690, 693, and 694) are shown in purple space-filling representation. Yellow, classical charge clamp residues. E355 neutralizes the positive dipole at the N terminus of the coactivator peptide, whereas K187 neutralizes the negative dipole at the C terminus. Orange, carboxylate group at C terminus of CAR. Green, K205, which forms a salt bridge with the C-terminal carboxylate to stabilize H12/AF2 in the active conformation. White, TCPOBOP docked in the ligand-binding cavity beneath H12. The loop between H11 and H12 is indicated and points to the junction between residues 345 and 346, the insertion site of three alanine residues.
FIG. 5.
Structural features required for constitutive activity of CAR. (A) CV-1 cells were cotransfected with different CAR expression plasmids, a luciferase reporter construct containing two copies of βRE2, and CMX-βgal. Cells were treated with ligands for 40 h before luciferase and β-galactosidase activities were measured. (B) Mammalian two-hybrid assay was performed by transfecting CV-1 cells with different expression plasmids for VP-CAR LBD, RXR LBD, Gal-SRC-1 (left panel), or Gal-SMRT (right panel) and the luciferase reporter UASGx4. Cells were treated for 40 h with ligands before luciferase and β-galactosidase activities were measured.
Similar articles
- The critical role of carboxy-terminal amino acids in ligand-dependent and -independent transactivation of the constitutive androstane receptor.
Andersin T, Väisänen S, Carlberg C. Andersin T, et al. Mol Endocrinol. 2003 Feb;17(2):234-46. doi: 10.1210/me.2002-0263. Mol Endocrinol. 2003. PMID: 12554751 - Antagonist- and inverse agonist-driven interactions of the vitamin D receptor and the constitutive androstane receptor with corepressor protein.
Lempiäinen H, Molnár F, Macias Gonzalez M, Peräkylä M, Carlberg C. Lempiäinen H, et al. Mol Endocrinol. 2005 Sep;19(9):2258-72. doi: 10.1210/me.2004-0534. Epub 2005 May 19. Mol Endocrinol. 2005. PMID: 15905360 - Regulatory Mechanics of Constitutive Androstane Receptors: Basal and Ligand-Directed Actions.
Pham B, Arons AB, Vincent JG, Fernandez EJ, Shen T. Pham B, et al. J Chem Inf Model. 2019 Dec 23;59(12):5174-5182. doi: 10.1021/acs.jcim.9b00695. Epub 2019 Dec 2. J Chem Inf Model. 2019. PMID: 31714771 - Ligand recognition by drug-activated nuclear receptors PXR and CAR: structural, site-directed mutagenesis and molecular modeling studies.
Poso A, Honkakoski P. Poso A, et al. Mini Rev Med Chem. 2006 Aug;6(8):937-47. doi: 10.2174/138955706777935008. Mini Rev Med Chem. 2006. PMID: 16918499 Review.
Cited by
- Strategies for developing pregnane X receptor antagonists: Implications from metabolism to cancer.
Chai SC, Wright WC, Chen T. Chai SC, et al. Med Res Rev. 2020 May;40(3):1061-1083. doi: 10.1002/med.21648. Epub 2019 Nov 28. Med Res Rev. 2020. PMID: 31782213 Free PMC article. Review. - Xenobiotic-sensing nuclear receptors involved in drug metabolism: a structural perspective.
Wallace BD, Redinbo MR. Wallace BD, et al. Drug Metab Rev. 2013 Feb;45(1):79-100. doi: 10.3109/03602532.2012.740049. Epub 2012 Dec 5. Drug Metab Rev. 2013. PMID: 23210723 Free PMC article. Review. - Regulation of PXR and CAR by protein-protein interaction and signaling crosstalk.
Oladimeji P, Cui H, Zhang C, Chen T. Oladimeji P, et al. Expert Opin Drug Metab Toxicol. 2016 Sep;12(9):997-1010. doi: 10.1080/17425255.2016.1201069. Epub 2016 Jun 23. Expert Opin Drug Metab Toxicol. 2016. PMID: 27295009 Free PMC article. Review. - Structural and functional similarity of amphibian constitutive androstane receptor with mammalian pregnane X receptor.
Mathäs M, Nusshag C, Burk O, Gödtel-Armbrust U, Herlyn H, Wojnowski L, Windshügel B. Mathäs M, et al. PLoS One. 2014 May 5;9(5):e96263. doi: 10.1371/journal.pone.0096263. eCollection 2014. PLoS One. 2014. PMID: 24797902 Free PMC article. - Retinoids activate RXR/CAR-mediated pathway and induce CYP3A.
Chen S, Wang K, Wan YJ. Chen S, et al. Biochem Pharmacol. 2010 Jan 15;79(2):270-6. doi: 10.1016/j.bcp.2009.08.012. Epub 2009 Aug 15. Biochem Pharmacol. 2010. PMID: 19686701 Free PMC article.
References
- Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965-968. - PubMed
- Brzozowski, A. M., A. C. Pike, Z. Dauter, R. E. Hubbard, T. Bonn, O. Engstrom, L. Ohman, G. L. Greene, J. A. Gustafsson, and M. Carlquist. 1997. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753-758. - PubMed
- Chen, H., R. J. Lin, R. L. Schiltz, D. Chakravarti, A. Nash, L. Nagy, M. L. Privalsky, Y. Nakatani, and R. M. Evans. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569-580. - PubMed
- Chen, J. D., and R. M. Evans. 1995. A transcriptional corepressor that interacts with nuclear hormone receptors. Nature 377:454-457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources