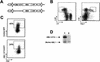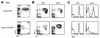Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation - PubMed (original) (raw)
Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation
Cristina G de Guzman et al. Mol Cell Biol. 2002 Aug.
Abstract
The t(8;21)(q22;q22) translocation, which fuses the ETO gene on human chromosome 8 with the AML1 gene on chromosome 21 (AML1-ETO), is one of the most frequent cytogenetic abnormalities associated with acute myelogenous leukemia (AML). It is seen in approximately 12 to 15% of AML cases and is present in about 40% of AML cases with a French-American-British classified M2 phenotype. We have generated a murine model of the t(8;21) translocation by retroviral expression of AML1-ETO in purified hematopoietic stem cells (HSC). Animals reconstituted with AML1-ETO-expressing cells recapitulate the hematopoietic developmental abnormalities seen in the bone marrow of human patients with the t(8;21) translocation. Primitive myeloblasts were increased to approximately 10% of bone marrow by 10 months posttransplant. Consistent with this observation was a 50-fold increase in myeloid colony-forming cells in vitro. Accumulation of late-stage metamyelocytes was also observed in bone marrow along with an increase in immature eosinophilic myelocytes that showed abnormal basophilic granulation. HSC numbers in the bone marrow of 10-month-posttransplant animals were 29-fold greater than in transplant-matched control mice, suggesting that AML1-ETO expression overrides the normal genetic control of HSC pool size. In summary, AMLI-ETO-expressing animals recapitulate many (and perhaps all) of the developmental abnormalities seen in human patients with the t(8;21) translocation, although the animals do not develop leukemia or disseminated disease in peripheral tissues like the liver or spleen. This suggests that the principal contribution of AML1-ETO to acute myeloid leukemia is the inhibition of multiple developmental pathways.
Figures
FIG. 1.
Retroviral transduction of murine hematopoietic stem cells. (A) Schematic diagram of MSCV retroviral constructs (control MSCV IRES GFP and MSCV _AML1_-ETO IRES GFP). (B) Gating used for sorting the HSC phenotype c-Kit+Sca-1+Lin− (where Lin represents a cocktail of antibodies to the mature blood cell antigens Mac-1, Gr-1, Ter119, B220, CD3, CD4, CD5, and CD8). (C) Flow cytometric analysis of HSC 24 h after retroviral transduction. Approximately 300 Ly-5.2+ HSC from control or AML1-ETO transductions were transplanted with a radioprotective dose of 2 × 105 Ly-5.1+ whole bone marrow cells into each Ly-5.1+ recipient animal. (D) Western blot analysis of GFP+ (lane 1) or GFP− (lane 2) myeloid scatter-gated cells FACS-sorted from the bone marrow of an 8-week-posttransplant AML1-ETO animal probed with a polyclonal anti-AML1 antibody.
FIG. 2.
Abnormal myelopoiesis and decreased B lymphopoiesis in AML1-ETO/GFP+ peripheral blood cells. (A) Flow cytometric analysis of peripheral blood cells from animals at 2.5 months posttransplantation stained with an antibody to the Ly-5.2 donor marker. Peripheral blood cells were gated as GFP− or GFP+ and analyzed for (B) simultaneous Mac-1 and Gr-1 or (C) B220 expression. Note the reduction in Mac-1loGr-1hi cells that represent mature neutrophils and an overrepresentation of Mac-1hiGr-1int cells in the AML1-ETO/GFP+ population compared to the GFP+ control. FACS plots are representative of all cocultured whole bone marrow transplants of control GFP (n = 21) and AML1-ETO-expressing (n = 26) mice and all purified HSC transplants of control GFP (n = 5) and AML1-ETO-expressing (n = 3) mice.
FIG. 3.
Abnormal myelopoiesis in AML1-ETO-expressing bone marrow cells. Flow cytometric analysis of bone marrow from a 10-month-posttransplant AML1-ETO mouse. (A) Bone marrow cells were gated on (panel 1) GFP− and (panel 2) AML1-ETO/GFP+ bone marrow cells and analyzed for expression of Mac-1 and Gr-1. The data are representative of all AML1-ETO-transplanted animals between 2 and 10 months posttransplant. The Mac-1/Gr-1 profile in panel 1 is identical to what is seen in bone marrow from control GFP animals. (B) Wright-Giemsa-stained cytospin preparation of AML1-ETO/GFP+, Mac-1hiGr-1int cells gated as shown in panel A (×100 magnification). Arrows indicate (a) a banded neutrophil and (b) a metamyelocyte. (C) Graded levels of AML1-ETO expression show distinct Mac-1/Gr-1 phenotypes in bone marrow. (D) Northern blot analysis of RNA isolated from GFP− and AML1-ETO/GFP+ bone marrow cells from a 3-month-posttransplant AML1-ETO animal. The blot was probed with a 3′ fragment of the C/EBPα cDNA and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe. Quantitation of transcript levels was done on a phosphoimager. (E) Wright-Giemsa staining of an eosinophil myelocyte, showing abnormal basophilic granulation from the bone marrow of a 10-month-posttransplant AML1-ETO animal.
FIG. 4.
Increase in myeloid colony-forming cells in AML1-ETO animals. (A) Myeloid scatter-gated cells were sorted as GFP− or AML1-ETO/GFP+ from a 10-month-old AML1-ETO mouse, and 1,000 cells from each population were plated in triplicate into M3434 methylcellulose medium (10 ng of murine recombinant interleukin-3, 10 ng of human recombinant interleukin-6, and 50 ng of murine recombinant stem cell factor per ml) supplemented with 0.5 ng of granulocyte-macrophage colony-stimulating factor (R & D Systems) per ml. (B) Three independent AML1-ETO animals at 2 and 10 months posttransplant were used in the analysis. Colonies were enumerated (1 colony of >200 cells) and characterized 10 days after plating. (C) Representative FACS plots of individual methylcellulose colonies stained with Mac-1 and Gr-1. Two representative plots are shown for each sample. (D) Cytospin preparations of GFP− and AML1-ETO/GFP+ colonies stained with Wright-Giemsa. Arrows indicate mature, segmented neutrophils among the GFP− cells that were not seen in any AML1-ETO-expressing colonies.
FIG. 5.
Expansion of hematopoietic stem cells in AML1-ETO mice. HSC analysis from a 10-month-posttransplant AML1-ETO mouse. Bone marrow cells were stained with c-Kit, lineage marker antibodies (see text), Sca-1, and the Ly-5.2 donor marker. The percentages of cells in individual gated populations are indicated.
FIG. 6.
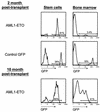
Delayed differentiation in AML1-ETO-expressing stem cells. The percentage of AML1-ETO-expressing (GFP+) cells in the stem cell population and in whole bone marrow was contrasted at early (2 months, n = 3) and late (10 months, n = 3) times postreconstitution. The ratio of GFP+ cells in the stem cell compartment and in the bone marrow of control GFP animals was similar to the ratio seen in older AML1-ETO animals.
FIG. 7.
AML1-ETO expression in stem cells is required for maintenance of abnormal myelopoiesis. Bone marrow from one primary recipient AML1-ETO animal was serially transplanted at a dose of 4 × 106 cells into each of four lethally irradiated secondary mice. Flow cytometric analysis of HSC in one of four secondary animals is shown at 5 weeks posttransplant. All secondary transplant animals received 114,000 AML1-ETO-expressing myeloid cells along with approximately 600 AML1-ETO/GFP+ HSC in the bone marrow inoculum. WBM, whole bone marrow.
Similar articles
- The ETO domain is necessary for the developmental abnormalities associated with AML1-ETO expression in the hematopoietic stem cell compartment in vivo.
de Guzman CG, Johnson A, Klug CA. de Guzman CG, et al. Blood Cells Mol Dis. 2003 Mar-Apr;30(2):201-6. doi: 10.1016/s1079-9796(03)00025-1. Blood Cells Mol Dis. 2003. PMID: 12732184 - Distinct classes of c-Kit-activating mutations differ in their ability to promote RUNX1-ETO-associated acute myeloid leukemia.
Nick HJ, Kim HG, Chang CW, Harris KW, Reddy V, Klug CA. Nick HJ, et al. Blood. 2012 Feb 9;119(6):1522-31. doi: 10.1182/blood-2011-02-338228. Epub 2011 Sep 21. Blood. 2012. PMID: 21937700 Free PMC article. - Oncogenic pathways of AML1-ETO in acute myeloid leukemia: multifaceted manipulation of marrow maturation.
Elagib KE, Goldfarb AN. Elagib KE, et al. Cancer Lett. 2007 Jun 28;251(2):179-86. doi: 10.1016/j.canlet.2006.10.010. Epub 2006 Nov 27. Cancer Lett. 2007. PMID: 17125917 Free PMC article. Review. - AML-1-ETO-Mediated erythroid inhibition: new paradigms for differentiation blockade by a leukemic fusion protein.
Choi Y, Elagib KE, Goldfarb AN. Choi Y, et al. Crit Rev Eukaryot Gene Expr. 2005;15(3):207-16. doi: 10.1615/critreveukargeneexpr.v15.i3.30. Crit Rev Eukaryot Gene Expr. 2005. PMID: 16390317 Review.
Cited by
- CBFbeta is critical for AML1-ETO and TEL-AML1 activity.
Roudaia L, Cheney MD, Manuylova E, Chen W, Morrow M, Park S, Lee CT, Kaur P, Williams O, Bushweller JH, Speck NA. Roudaia L, et al. Blood. 2009 Mar 26;113(13):3070-9. doi: 10.1182/blood-2008-03-147207. Epub 2009 Jan 29. Blood. 2009. PMID: 19179469 Free PMC article. - Disruption of the NHR4 domain structure in AML1-ETO abrogates SON binding and promotes leukemogenesis.
Ahn EY, Yan M, Malakhova OA, Lo MC, Boyapati A, Ommen HB, Hines R, Hokland P, Zhang DE. Ahn EY, et al. Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):17103-8. doi: 10.1073/pnas.0802696105. Epub 2008 Oct 24. Proc Natl Acad Sci U S A. 2008. PMID: 18952841 Free PMC article. - Development and Validation of UPLC-MS/MS Method for Determination of Enasidenib in Rat Plasma and Its Pharmacokinetic Application.
Li SL, Zhu YL, Zhang Y, Liu SH, Wang XD, Qiu XJ. Li SL, et al. Int J Anal Chem. 2020 Mar 31;2020:5084127. doi: 10.1155/2020/5084127. eCollection 2020. Int J Anal Chem. 2020. PMID: 32292480 Free PMC article. - Maintenance of leukemia-initiating cells is regulated by the CDK inhibitor Inca1.
Bäumer N, Bäumer S, Berkenfeld F, Stehling M, Köhler G, Berdel WE, Müller-Tidow C, Tschanter P. Bäumer N, et al. PLoS One. 2014 Dec 19;9(12):e115578. doi: 10.1371/journal.pone.0115578. eCollection 2014. PLoS One. 2014. PMID: 25525809 Free PMC article. - Mouse models as tools to understand and study BCR-ABL1 diseases.
Koschmieder S, Schemionek M. Koschmieder S, et al. Am J Blood Res. 2011;1(1):65-75. Epub 2011 Jun 7. Am J Blood Res. 2011. PMID: 22432067 Free PMC article.
References
- Andrieu, V., I. Radford-Weiss, X. Troussard, C. Chane, F. Valensi, M. Guesnu, E. Haddad, F. Viguier, F. Dreyfus, B. Varet, G. Flandrin, and E. Macintyre. 1996. Molecular detection of t(8;21)/AML1-ETO in AML M1/M2: correlation with cytogenetics, morphology and immunophenotype. Br. J. Haematol. 92:855-865. - PubMed
- Bartram, C. R., W. D. Ludwig, W. Hiddemann, J. Lyons, M. Buschle, J. Ritter, J. Harbott, A. Frohlich, and J. W. Janssen. 1989. Acute myeloid leukemia: analysis of ras gene mutations and clonality defined by polymorphic X-linked loci. Leukemia 3:247-256. - PubMed
- Beghini, A., P. Peterlongo, C. B. Ripamonti, L. Larizza, R. Cairoli, E. Morra, and C. Mecucci. 2000. c-kit mutations in core binding factor leukemias. Blood 95:726-727. - PubMed
- Bonnet, D., and J. E. Dick. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3:730-737. - PubMed
- Bravo, J., Z. Li, N. A. Speck, and A. J. Warren. 2001. The leukemia-associated AML1 (Runx1)-CBF beta complex functions as a DNA-induced molecular clamp. Nat. Struct. Biol. 8:371-378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases