The cytoplasmic filaments of the nuclear pore complex are dispensable for selective nuclear protein import - PubMed (original) (raw)
The cytoplasmic filaments of the nuclear pore complex are dispensable for selective nuclear protein import
Tobias C Walther et al. J Cell Biol. 2002.
Abstract
The nuclear pore complex (NPC) mediates bidirectional macromolecular traffic between the nucleus and cytoplasm in eukaryotic cells. Eight filaments project from the NPC into the cytoplasm and are proposed to function in nuclear import. We investigated the localization and function of two nucleoporins on the cytoplasmic face of the NPC, CAN/Nup214 and RanBP2/Nup358. Consistent with previous data, RanBP2 was localized at the cytoplasmic filaments. In contrast, CAN was localized near the cytoplasmic coaxial ring. Unexpectedly, extensive blocking of RanBP2 with gold-conjugated antibodies failed to inhibit nuclear import. Therefore, RanBP2-deficient NPCs were generated by in vitro nuclear assembly in RanBP2-depleted Xenopus egg extracts. NPCs were formed that lacked cytoplasmic filaments, but that retained CAN. These nuclei efficiently imported nuclear localization sequence (NLS) or M9 substrates. NPCs lacking CAN retained RanBP2 and cytoplasmic filaments, and showed a minor NLS import defect. NPCs deficient in both CAN and RanBP2 displayed no cytoplasmic filaments and had a strikingly immature cytoplasmic appearance. However, they showed only a slight reduction in NLS-mediated import, no change in M9-mediated import, and were normal in growth and DNA replication. We conclude that RanBP2 is the major nucleoporin component of the cytoplasmic filaments of the NPC, and that these filaments do not have an essential role in importin alpha/beta- or transportin-dependent import.
Figures
Figure 3.
Immunodepletion of CAN/Nup214 and RanBP2 from Xenopus laevis egg extracts. (A) Immunoblotting confirms specificity of affinity-purified antibodies for Xenopus CAN/Nup214 and RanBP2/Nup358. Proteins of 25 manually isolated Xenopus oocyte nuclei (lane 1) and 13,000 g supernatant of Xenopus egg extract from 4–5 cells (lane 2–4) were separated by SDS-PAGE and used for immunodetection of RanBP2/358V (lanes 1 and 2), RanBP2/358F (lane 3), and CAN (lane 4) by enhanced chemiluminescence reaction. Note that independent of exposure time, RanBP2 is the only protein immunodetected in egg extracts. In the nuclear fraction, a minor cross-reaction with an unknown protein of ∼40 kD is seen only after prolonged exposure (unpublished data). Positions of marker proteins of 250, 150, 100, 75, 50, 37, and 25 kD are given at the right margins. (B) Monoclonal 414 immunoblot of undepleted (lane 1), or immunodepleted (lanes 2–7) fractionated Xenopus egg extracts as indicated above the lanes. (Lane 8) Fractionated membranes. Positions of RanBP2/Nup358, CAN/Nup214, Nup153, and p62 are indicated on the left.
Figure 1.
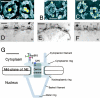
Isolated Xenopus oocyte NEs were fixed and labeled with affinity-purified anti-CAN or anti-RanBP2 antibodies, followed by 10-nm gold-conjugated secondary antibodies, and analyzed by FEISEM and TEM. (A–C) Representative FEISEM images of the cytoplasmic face of the NE labeled with 10-nm gold particles (artificially colored yellow) recognizing (A) anti-CAN/Nup214 antibodies; (B) anti-RanBP2/Nup358F antibodies; and (C) anti-RanBP2/Nup358V antibodies. Bars, 100 nm. (D–F) Representative TEM images of thin sections cut through the NE decorated with 10-nm gold labeling (D) anti-CAN/Nup214 antibodies; (E) anti-RanBP2/Nup358F antibodies; and (F) anti-RanBP2/Nup358V antibodies. CF, cytoplasmic filaments; NP, nuclear pore; ONM, outer nuclear membrane; INM, inner nuclear membrane; N, nucleus; C, cytoplasm. Bar, 100 nm. (G) Diagrammatical representation of the NPC summarizing the localization data for CAN/Nup214 and RanBP2/Nup358 using anti-RanBP2/Nup358F (green), anti-RanBP2/Nup358V (red), and anti-CAN/Nup214 (blue) antibodies. Putative positioning of substructures of the NPC are shaded in gray. Sizes and positions of substructures were derived from (Akey, 1989; Jarnik and Aebi, 1991; Akey and Radermacher, 1993; Goldberg and Allen, 1996) and our own measurements (unpublished data). Bar, 50 nm. Error bars represent standard deviations of the means.
Figure 2.
Antibodies specific for Xenopus RanBP2 label NPC-associated cytoplasmic filaments in vivo but do not prevent subsequent nuclear import of 10-nm gold particles coated with BSA-SV40NLS. (A) Transmission electron micrographs of ultrathin cross sections through NPCs of Xenopus oocytes after initial microinjection of 5-nm gold-coupled RanBP2/Nup358V antibodies and subsequent injection of 10-nm gold-coupled BSA-NLS. In all images, cytoplasm (CYT) and nuclear interior (NUC) adjacent to the nuclear envelope are oriented to the top and bottom, respectively. In A.3, the 5-nm gold grains which are coupled to the RanBP2 antibodies decorating the NPC-attached cytoplasmic fibrils are highlighted with blue circles; red circles mark the 10-nm gold grains coated with BSA-NLS. (B) Relative distribution of 5- (blue) and 10-nm gold particles (red) with respect to an idealistic NPC in cross section. Signals were assembled from 15 representative NPC images. Note that the distance between the peak distribution of the 5-nm gold particles and the central midplane of the NPC is ∼70 nm, whereas an area more proximal to the midplane (distance from 45 to 0 nm) is essentially devoid of 5-nm gold grains. Note further that 10-nm gold particles most proximal to the midplane are found only near the central “channel” region of NPC. Bars, 50 nm. (C) Schematic representation of sequential injections of antibodies and transport substrate into stage VI oocytes. Primary injections (t0) with 5-nm gold anti-RanBP2, or with buffer alone for mock injections, were at two opposite sites of the equatorial borderline between the oocyte's hemispheres. Subsequent injections of 10-nm-gold BSA-SV40NLS (t16) were into the vegetal hemisphere. Oocytes were further incubated and processed for electron microscopy.
Figure 4.
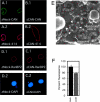
CAN/Nup214-deficient NPCs. (A–D). Nuclei that lack CAN/Nup214 retain RanBP2/Nup358. In vitro formed nuclei from mock-depleted (A and D) or CAN/Nup214 depleted (B and C) Xenopus egg extracts were stained with anti-CAN/Nup214 (A.1 and A.2), anti-RanBP2/Nup358 (C.1 and C.2), monoclonal 414 (A.2 and B.2), and DAPI (C.2 and D.2). (E) CAN/Nup214-deficient NPCs retain cytoplasmic filaments. Nuclei formed from CAN/Nup214-depleted egg extracts were imaged from the cytoplasmic side using FEISEM. Arrowheads indicate cytoplasmic filaments. Bar, 100 nm. (F) CAN/Nup214-deficient nuclei show nuclear import of BSA-NLS to be reduced to 75% of control levels. In vitro assembled nuclei from mock or CAN/Nup214 (ΔCAN)-depleted egg extracts were incubated with FITC- and TRITC-labeled BSA–SLN. After 30 min of import, nuclei were fixed and the intranuclear FITC signal quantified. A minority of nuclei that showed TRITC signal were excluded from the analysis. Bars represent standard errors of means.
Figure 5.
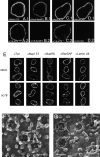
RanBP2/Nup358-deficient NPCs. (A–E) Nuclei that lack RanBP2/Nup358 retain CAN/Nup214 but lack RanGAP1. In vitro formed nuclei from mock-depleted (A.1, A.2, C.1, D.1, and E.1) or RanBP2/Nup358-depleted (B.1, B.2, C.2, D.2, and E.2) Xenopus egg extracts were stained with anti-Nup358V (A.1 and B.1), monoclonal 414 (C.1 and C.2), anti-RanGAP1 (D.1 and D.2), and anti-CAN/Nup214 (E.1 and E.2), and DAPI (A.2 and B.2). Representative images are shown. (F–G) RanBP2/Nup358 deficient NPCs lack cytoplasmic filaments. Nuclei formed from mock-depleted (F and F′) or RanBP2/Nup358-depleted (G and G′) egg extracts were imaged from the cytoplasmic side using FEISEM. CF, cytoplasmic filament; CR, cytoplasmic ring; R, ribosome. Bars, 100 nm. (H) RanBP2/Nup358-deficient nuclei show normal nuclear import of BSA-NLS. In vitro assembled nuclei from mock- (ΔMock) or RanBP2/Nup358- (ΔBP2) depleted egg extracts were assayed for nuclear import as described in Fig. 4.
Figure 6.
CAN/Nup214- and RanBP2/Nup358-deficient NPCs. (A–D) Formation of nuclei that lack both CAN/Nup214 and RanBP2/Nup358. In vitro formed nuclei from mock-depleted (A.1, B.1, C.1, D.1) or CAN/RanBP2-depleted (A.2, B.2, C.2, D.2) Xenopus egg extracts were stained with monoclonal 414 (A and C), anti-Nup358V (B), and anti-CAN/Nup214 (D). Representative images are shown. (E) CAN and RanBP2-deficient nuclei retain nuclear NPC components and a nuclear lamina. Assembled nuclei as above were immunostained with antibodies to Tpr, Nup153, Nup98, RanGAP1, and Lamin LIII. (F and G) RanBP2/Nup358-deficient NPCs lack cytoplasmic filaments and show an immature cytoplasmic ring. Nuclei formed from mock- (F) or CAN/RanBP2-depleted (G) egg extracts were imaged from the cytoplasmic side using FEISEM. CF, cytoplasmic filaments; CTR, cytoplasmic thin rings; SR, star ring. Bar, 100 nm.
Figure 7.
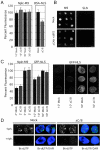
Nuclear import in CAN- and RanBP2-deficient nuclei. (A) CAN/RanBP2-deficient nuclei show normal nuclear import of Nplc-M9 and slightly reduced import of BSA-NLS. In vitro assembled nuclei from mock- (Mock) or CAN/RanBP2- (ΔC/B) depleted egg extracts were incubated with FITC-labeled Nplc-M9 or BSA-NLS. After 10 and 30 min (M9) or 40 min (NLS) of import, nuclei were fixed and intranuclear FITC signal was quantified as in Fig. 4. (B) CAN/RanBP2-deficient nuclei are not hyperpermeable. Random samples of nuclei from A. (M9) were analyzed for exclusion of BSA–SLN. No difference regarding nuclear exclusion of BSA–SLN was observed between mock-depleted and CAN/RanBP2-deficient nuclei. (C) Initial rates of transportin- and importin α/β-mediated import are unaffected by absence of CAN and RanBP2. Mock- or CAN/RanBP2-defecient nuclei were incubated with FITC-labeled nucleoplasmic core M9 (Nplc-M9) or GFP-NLS. After 2 and 5 min (M9) or 10 and 30 min (NLS) of import, nuclei were fixed and intranuclear FITC signal was quantified as in Fig. 4. Representative images are shown to the left. (D) CAN and RanBP2-deficient nuclei are DNA replication competent. Mock- or CAN/RanBP2-deficient nuclei were assembled in the presence of 40 μM 21-biotin dUTP (Bt-dUTP) and in the presence or absence of 14 μM aphidicolin (Aph.) as indicated. Nuclei were fixed and stained with Alexa 488–labeled streptavidin (green) and DAPI (blue). Bars, 10 μm.
Similar articles
- Mediators of nuclear protein import target karyophilic proteins to pore complexes of cytoplasmic annulate lamellae.
Cordes VC, Rackwitz HR, Reidenbach S. Cordes VC, et al. Exp Cell Res. 1997 Dec 15;237(2):419-33. doi: 10.1006/excr.1997.3806. Exp Cell Res. 1997. PMID: 9434638 - The nucleoporin Nup358/RanBP2 promotes nuclear import in a cargo- and transport receptor-specific manner.
Wälde S, Thakar K, Hutten S, Spillner C, Nath A, Rothbauer U, Wiemann S, Kehlenbach RH. Wälde S, et al. Traffic. 2012 Feb;13(2):218-33. doi: 10.1111/j.1600-0854.2011.01302.x. Epub 2011 Nov 21. Traffic. 2012. PMID: 21995724 - The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins.
Walther TC, Fornerod M, Pickersgill H, Goldberg M, Allen TD, Mattaj IW. Walther TC, et al. EMBO J. 2001 Oct 15;20(20):5703-14. doi: 10.1093/emboj/20.20.5703. EMBO J. 2001. PMID: 11598013 Free PMC article. - The nuclear pore complex.
Davis LI. Davis LI. Annu Rev Biochem. 1995;64:865-96. doi: 10.1146/annurev.bi.64.070195.004245. Annu Rev Biochem. 1995. PMID: 7574503 Review. - Structural dynamics of the nuclear pore complex.
Sakiyama Y, Panatala R, Lim RYH. Sakiyama Y, et al. Semin Cell Dev Biol. 2017 Aug;68:27-33. doi: 10.1016/j.semcdb.2017.05.021. Epub 2017 Jun 1. Semin Cell Dev Biol. 2017. PMID: 28579449 Review.
Cited by
- Systematic analysis of barrier-forming FG hydrogels from Xenopus nuclear pore complexes.
Labokha AA, Gradmann S, Frey S, Hülsmann BB, Urlaub H, Baldus M, Görlich D. Labokha AA, et al. EMBO J. 2013 Jan 23;32(2):204-18. doi: 10.1038/emboj.2012.302. Epub 2012 Nov 30. EMBO J. 2013. PMID: 23202855 Free PMC article. - Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity.
Schmidt HB, Görlich D. Schmidt HB, et al. Elife. 2015 Jan 6;4:e04251. doi: 10.7554/eLife.04251. Elife. 2015. PMID: 25562883 Free PMC article. - Porcine Reproductive and Respiratory Syndrome Virus Nonstructural Protein 1 Beta Interacts with Nucleoporin 62 To Promote Viral Replication and Immune Evasion.
Ke H, Han M, Kim J, Gustin KE, Yoo D. Ke H, et al. J Virol. 2019 Jun 28;93(14):e00469-19. doi: 10.1128/JVI.00469-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31043527 Free PMC article. - Increased Gene Expression in Cultured BEAS-2B Cells Treated with Metal Oxide Nanoparticles.
Park EJ, Park K. Park EJ, et al. Toxicol Res. 2009 Dec;25(4):195-201. doi: 10.5487/TR.2009.25.4.195. Epub 2009 Dec 30. Toxicol Res. 2009. PMID: 32038838 Free PMC article. - Identification and characterization of nuclear pore complex components in Arabidopsis thaliana.
Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I. Tamura K, et al. Plant Cell. 2010 Dec;22(12):4084-97. doi: 10.1105/tpc.110.079947. Epub 2010 Dec 28. Plant Cell. 2010. PMID: 21189294 Free PMC article.
References
- Allen, T.D., J.M. Cronshaw, S. Bagley, E. Kiseleva, and M.W. Goldberg. 2000. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell Sci. 113:1651–1659. - PubMed
- Arts, G.J., L. Englmeier, and I.W. Mattaj. 1997. Energy- and temperature-dependent in vitro export of RNA from synthetic nuclei. Biol. Chem. 378:641–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous