An HRD/DER-independent ER quality control mechanism involves Rsp5p-dependent ubiquitination and ER-Golgi transport - PubMed (original) (raw)
An HRD/DER-independent ER quality control mechanism involves Rsp5p-dependent ubiquitination and ER-Golgi transport
Cole M Haynes et al. J Cell Biol. 2002.
Abstract
We have identified a new pathway of ER-associated degradation in Saccharomyces cerevisiae that functions separately from the HRD/DER pathway comprised of Hrd1p, Hrd3p, Der1p, and Ubc7p. This pathway, termed Hrd1p independent-proteolysis (HIP), is capable of recognizing and degrading both lumenal (CPY* and PrA*), and integral membrane proteins (Sec61-2p) that misfold in the ER. CPY* overexpression likely saturates the HRD/DER pathway and activates the HIP pathway, so the slowed degradation kinetics of CPY* in a hrd1 Delta strain is restored to a wild-type rate when CPY* is overexpressed. Substrates of HIP require vesicular trafficking between the ER and Golgi apparatus before degradation by the ubiquitin-proteasome system. Ubiquitination of HIP substrates does not involve the HRD/DER pathway ubiquitin ligase Hrd1p, but instead uses another ubiquitin ligase, Rsp5p. HIP is regulated by the unfolded protein response as Ire1p is necessary for the degradation of CPY* when overexpressed, but not when CPY* is expressed at normal levels. Both the HIP and HRD/DER pathways contribute to the degradation of CPY*, and only by eliminating both is CPY* degradation completely blocked.
Figures
Figure 1.
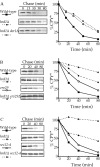
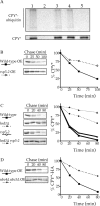
CPY* degradation is completely blocked upon inhibition of both the HRD/DER pathway and ER-Golgi transport. _hrd1_Δ (KHY171), _hrd1_Δ _der1_Δ (KHY237), _erv29_Δ (KHY270), _erv29_Δ _hrd1_Δ (KHY279), sec12–4 (KHY306), _sec12–4 hrd1_Δ (KHY308), and wild-type (KHY163) cells were radiolabeled, chased, and CPY* immunoprecipitated at various times. Samples were separated by SDS-PAGE, visualized by fluorography, and quantitated using a Phosphorimager as performed previously (Hill and Cooper, 2000). (A) HRD/DER pathway–deficient strains (_hrd1_Δ, _hrd1_Δ _der1_Δ) and wild-type cells expressing single copy levels of CPY*. (B) _hrd1_Δ, _erv29_Δ, _hrd1_Δ _erv29_Δ, or wild-type strains expressing single copy levels of CPY*. (C) _hrd1_Δ, sec12–4, _hrd1_Δ sec12–4, or wild-type strains expressing single copy levels of CPY* (34°C).
Figure 2.
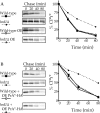
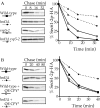
CPY* and PrA* can be degraded independently of the HRD/DER pathway. _hrd1_Δ and wild-type strains were radiolabeled, chased, and CPY* (A and B) immunoprecipitated at various times. Samples were analyzed as in Fig. 1. (A) Wild-type (KHY163) and _hrd1_Δ (KHY171) strains expressing single copy or overexpressed (OE) levels of CPY* (pAC453). (B) Wild-type (KHY163) and _hrd1_Δ (KHY171) strains expressing CPY* with and without overexpressed PrA*–HA (pAC540) present.
Figure 3.
The inhibition of ER-Golgi transport ( erv29 Δ) but not the HRD/DER pathway (hrd1 Δ ) significantly slows the degradation of overexpressed (OE) CPY*. erv29_Δ (KHY270), hrd1_Δ (KHY171), _hrd1_Δ _erv29_Δ (KHY279), and wild-type (KHY163) cells were analyzed as in Fig. 1. (A) _erv29_Δ and wild-type strains expressing either single copy or overexpressed (OE) levels of CPY* (pAC453). (B) Wild-type, _hrd1_Δ, _erv29_Δ, and _hrd1_Δ _erv29_Δ strains overexpressing (OE) CPY*. (C) Wild-type, _hrd1_Δ, and _hrd1_Δ _erv29_Δ strains either expressing single copy or overexpressed levels of CPY*. (D) _hrd1_Δ (KHY171) cells overexpressing CPY* were radiolabeled and CPY* immunoprecipitated. The precipitates were reimmunoprecipitated with either CPY* or α1,6-mannose antibodies.
Figure 4.
Overexpressed (OE) CPY* is degraded independently of the vacuole and is not secreted from the cell. (A) _hrd1_Δ (KHY171) and _hrd1_Δ _pep4_Δ (KHY265) strains were analyzed as in Fig. 1. _hrd1_Δ PEP4 and _hrd1_Δ _pep4_Δ strains expressing single copy or overexpressed levels of CPY* (pAC519). (B) _hrd1_Δ (KHY171) and wild-type (KHY163) cells overexpressing (OE) CPY* and _vps10_Δ (AACY9) cells expressing CPY were radiolabeled, chased, and CPY* or CPY was immunoprecipitated from intracellular (I) and extracellular (E) fractions as described previously (Cooper and Stevens, 1996). Samples were visualized by fluorography.
Figure 5.
The proteasome is responsible for the degradation of overexpressed (OE) CPY*. pre1 pre2 (KHY293) and wild-type (KHY292) strains expressing single copy (pAC446) or overexpressed levels of CPY* (pAC519) were analyzed as in Fig. 1.
Figure 6.
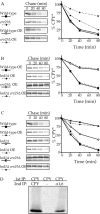
Rsp5p is the ubiquitin ligase of the HIP pathway and is responsible for the ubiquitination of CPY* in HRD/DER pathway–deficient cells. (A) Ubiquitination of CPY* in wild-type (lane 1), _prc1_Δ hrd1_Δ (lane 2), hrd1_Δ (lane 3), rsp5–2 (lane 4), and _hrd1_Δ rsp5–2 (lane 5) cells was assayed by radiolabeling at 37°C followed by sequential immunoprecipitations first with anti-CPY antibodies and then with anti–ubiquitin-HA antibodies. (B and C) rsp5–2 (KHY355), _hrd1_Δ (KHY171), _hrd1_Δ rsp5–2 (KHY359), and wild-type (KHY163) strains were radiolabeled, chased (at 37°C), and CPY* immunoprecipitated at various times. Samples were analyzed as in Fig. 1. (B) Wild-type and rsp5–2 cells overexpressing (OE) CPY* (pAC453). (C) Wild-type, _hrd1_Δ, rsp5–2, and _hrd1_Δ rsp5–2 expressing single copy levels of CPY*. (D) Wild-type (RH448) and _ubc4_Δ _ubc5_Δ (RH3097) overexpressing (OE) CPY*–HA were radiolabeled (at 30°C), chased, and CPY* immunoprecipitated.
Figure 7.
The integral membrane ERQC substrate Sec61–2p-HA can also be degraded by the HIP pathway. _hrd1_Δ (KHY171), rsp5–2 (KHY355), _hrd1_Δ rsp5–2 (KHY359), and wild-type (KHY163) cells were radiolabeled at 37°C (A) and 30°C (B), chased, Sec61–2p-HA immunoprecipitated at various times, and analyzed as in Fig. 1. (A) Wild-type, _hrd1_Δ, rsp5–2, and _hrd1_Δ rsp5–2 expressing Sec61–2p-HA (pAC460; Caldwell et al., 2001). (B) _prc1_Δ (KHY298) and _prc1_Δ _hrd1_Δ (KHY299) cells, in the absence of CPY*, expressing Sec61–2p-HA (pAC460) and wild-type (KHY163) and _hrd1_Δ (KHY171) in the presence of overexpressed (OE) CPY* expressing Sec61–2p-HA.
Figure 8.
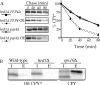
The UPR is required for efficient degradation of overexpressed (OE) CPY*. _ire1_Δ (KHY280) and wild-type (KHY163) cells expressing single copy and overexpressed levels (pAC453) of CPY* were analyzed as in Fig. 1.
Figure 9.
Summary: ERQC consists of two degradative mechanisms, the HIP and the HRD/DER pathway. Substrates destined for the HRD/DER pathway are recognized as being misfolded or unassembled, and are retained in the ER before being retrotranslocated through the translocon to the cytosol where the Hrd3p-regulated ubiquitin ligase (Hrd1p) attaches ubiquitin to the substrate and it is subsequently degraded by the 26S proteasome. The mechanism described here (HIP) eliminates misfolded proteins from the ER in a manner that requires vesicular transport from the ER to the Golgi apparatus involving Sec12p and the putative CPY* cargo receptor, Erv29p. Once in the cis-Golgi compartment, CPY* is modified by α1,6-mannose addition before ubiquitination by the ubiquitin ligase Rsp5p and subsequent degradation by the proteasome. Where the misfolded protein enters the cytosol and is exposed to Rsp5p is not clear, however, two locations seem possible: (1) it may enter the cytosol from the Golgi apparatus by an unknown mechanism, or (2) it may be transported back to the ER where it presumably would enter the cytosol through the translocon.
Similar articles
- In vivo action of the HRD ubiquitin ligase complex: mechanisms of endoplasmic reticulum quality control and sterol regulation.
Gardner RG, Shearer AG, Hampton RY. Gardner RG, et al. Mol Cell Biol. 2001 Jul;21(13):4276-91. doi: 10.1128/MCB.21.13.4276-4291.2001. Mol Cell Biol. 2001. PMID: 11390656 Free PMC article. - Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation.
Plemper RK, Bordallo J, Deak PM, Taxis C, Hitt R, Wolf DH. Plemper RK, et al. J Cell Sci. 1999 Nov;112 ( Pt 22):4123-34. doi: 10.1242/jcs.112.22.4123. J Cell Sci. 1999. PMID: 10547371 - Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins.
Bordallo J, Plemper RK, Finger A, Wolf DH. Bordallo J, et al. Mol Biol Cell. 1998 Jan;9(1):209-22. doi: 10.1091/mbc.9.1.209. Mol Biol Cell. 1998. PMID: 9437001 Free PMC article. - Versatile role of the yeast ubiquitin ligase Rsp5p in intracellular trafficking.
Belgareh-Touzé N, Léon S, Erpapazoglou Z, Stawiecka-Mirota M, Urban-Grimal D, Haguenauer-Tsapis R. Belgareh-Touzé N, et al. Biochem Soc Trans. 2008 Oct;36(Pt 5):791-6. doi: 10.1042/BST0360791. Biochem Soc Trans. 2008. PMID: 18793138 Review. - Endoplasmic reticulum degradation: reverse protein flow of no return.
Sommer T, Wolf DH. Sommer T, et al. FASEB J. 1997 Dec;11(14):1227-33. doi: 10.1096/fasebj.11.14.9409541. FASEB J. 1997. PMID: 9409541 Review.
Cited by
- Respiratory metabolism and calorie restriction relieve persistent endoplasmic reticulum stress induced by calcium shortage in yeast.
Busti S, Mapelli V, Tripodi F, Sanvito R, Magni F, Coccetti P, Rocchetti M, Nielsen J, Alberghina L, Vanoni M. Busti S, et al. Sci Rep. 2016 Jun 16;6:27942. doi: 10.1038/srep27942. Sci Rep. 2016. PMID: 27305947 Free PMC article. - Hsp40/70/110 chaperones adapt nuclear protein quality control to serve cytosolic clients.
Prasad R, Xu C, Ng DTW. Prasad R, et al. J Cell Biol. 2018 Jun 4;217(6):2019-2032. doi: 10.1083/jcb.201706091. Epub 2018 Apr 13. J Cell Biol. 2018. PMID: 29653997 Free PMC article. - Limited ER quality control for GPI-anchored proteins.
Sikorska N, Lemus L, Aguilera-Romero A, Manzano-Lopez J, Riezman H, Muñiz M, Goder V. Sikorska N, et al. J Cell Biol. 2016 Jun 20;213(6):693-704. doi: 10.1083/jcb.201602010. J Cell Biol. 2016. PMID: 27325793 Free PMC article. - The ubiquitin-proteasome system regulates the stability of neuronal nicotinic acetylcholine receptors.
Rezvani K, Teng Y, De Biasi M. Rezvani K, et al. J Mol Neurosci. 2010 Jan;40(1-2):177-84. doi: 10.1007/s12031-009-9272-x. Epub 2009 Aug 20. J Mol Neurosci. 2010. PMID: 19693707 Free PMC article. - Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class I major histocompatibility complex products.
Tirosh B, Iwakoshi NN, Lilley BN, Lee AH, Glimcher LH, Ploegh HL. Tirosh B, et al. J Virol. 2005 Mar;79(5):2768-79. doi: 10.1128/JVI.79.5.2768-2779.2005. J Virol. 2005. PMID: 15708995 Free PMC article.
References
- Bays, N.W., R.G. Gardner, L.P. Seelig, C.A. Joazeiro, and R.Y. Hampton. 2001. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat. Cell Biol. 3:24–29. - PubMed
- Belden, W.J., and C. Barlowe. 2001. Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science. 294:1528–1531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous