Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells - PubMed (original) (raw)
Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells
Jun Chung et al. J Cell Biol. 2002.
Abstract
We define a novel mechanism by which integrins regulate growth factor expression and the survival of carcinoma cells. Specifically, we demonstrate that the alpha 6 beta 4 integrin enhances vascular endothelial growth factor (VEGF) translation in breast carcinoma cells. The mechanism involves the ability of this integrin to stimulate the phosphorylation and inactivation of 4E-binding protein (4E-BP1), a translational repressor that inhibits the function of eukaryotic translation initiation factor 4E (eIF-4E). The regulation of 4E-BP1 phosphorylation by alpha 6 beta 4 derives from the ability of this integrin to activate the PI-3K-Akt pathway and, consequently, the rapamycin-sensitive kinase mTOR that can phosphorylate 4E-BP1. Importantly, we show that this alpha 6 beta 4-dependent regulation of VEGF translation plays an important role in the survival of metastatic breast carcinoma cells by sustaining a VEGF autocrine signaling pathway that involves activation of PI-3K and Akt. These findings reveal that integrin-mediated activation of PI-3K-Akt is amplified by integrin-stimulated VEGF expression and they provide a mechanism that substantiates the reported role of alpha 6 beta 4 in carcinoma progression.
Figures
Figure 1.
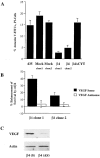
The α6β4-mediated survival of breast carcinoma cells is VEGF dependent. (A) Parental, mock (clone 1, 6D2; clone 2, 6D7), β4-ΔCYT–expressing (cytoplasmic tail deletion mutant), and β4 integrin–expressing (clone 1, 3A7; clone 2, 5B3) MDA-MB-435 subclones were maintained in low serum (0.5% FBS) medium for 24 h. To assess the level of apoptosis, these cells were stained with annexin V–FITC and propidium iodide (PI), and analyzed on a Becton Dickinson flow cytometer using CellQuest software. The percentage of annexin-positive, PI-negative cells (± SD) is indicated. Results were obtained from three independent experiments. Apoptosis was minimal in the presence of 10% FBS (unpublished data). (B) Mock-transfected clone 6D7 and β4 integrin– (clone 1, 3A7; clone 2, 5B3) expressing MDA-MB-435 subclones were transiently transfected with VEGF sense or antisense oligonucleotides and maintained in low serum (0.5% FBS) medium. After 24 h, the level of apoptosis in these cells was assessed as described above. The data are presented as the mean difference (± SD) in annexin positivity between mock-transfected and α6β4-expressing MDA-MB-435 cells. Similar results were observed in two separate experiments. (C) The relative amount of VEGF protein in extracts obtained from the MDA-MB-435/β4 cells transfected with either the VEGF sense (S) or antisense (AS) oligonucleotide was determined by immunoblotting using a polyclonal anti-VEGF immune serum.
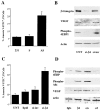
Figure 2.
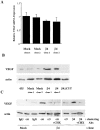
Expression of the α6β4 integrin increases VEGF protein but not steady-state mRNA. (A) The amount of VEGF mRNA in extracts obtained from mock- (clone 1, 6D2; clone 2, 6D7) and β4 integrin– (clone 1, 3A7; clone 2, 5B3) transfected MDA-MB-435 subclones was quantified by real-time PCR. The data are presented as the mean ratio of VEGF to β-actin mRNA (± SD) obtained from triplicate samples. (B) Parental (435), mock (clone 1, 6D2; clone 2, 6D7), β4-ΔCYT–expressing (clone 1E10), and β4 integrin–expressing (clone 1: 3A7, clone 2: 5B3) MDA-MB-435 subclones were cultured in low serum (0.5% FBS) medium for 24 h. Extracts of these cells containing equivalent amounts of protein were analyzed for their relative expression of VEGF and actin by immunoblotting. Similar results were observed in four independent experiments. (C) Mock (clone 6D7) and β4 integrin–expressing (clone 3A7) MDA-MB-435 subclones were maintained in low serum (0.5% FBS) medium for 24 h. These cells were detached with trypsin and incubated with integrin-specific antibodies (α6 integrin, 2B7; β4 integrin, A9; α5 integrin, Sam1) or IgG for 30 min in suspension and allowed to adhere on anti-IgG–coated plates for 60 min before lysis. In addition, cells were preincubated in cycloheximide (CHX) at a concentration of 10 μg/ml for 30 min and then incubated with either the α6 or β4 integrin antibodies in the presence of cycloheximide. Extracts of these cells containing equivalent amounts of protein were analyzed for their relative expression of VEGF and actin by immunoblotting. Similar results were observed in two independent experiments.
Figure 3.
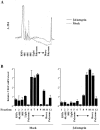
Polysome analysis of VEGF mRNA. (A) The distribution of RNA from MDA-MB-435/β4 and mock transfectants that had been fractionated on sucrose gradients as described in the Materials and methods was determined by measuring the A254 of each fraction. (B) The relative VEGF mRNA content of each sucrose gradient fraction was measured by real-time PCR as described in the Materials and methods. Fraction 1 contains unbound RNA present in the ribonucleoprotein fraction, fraction 2 contains 40S and 60S monosomes, fraction 3 contains 80S monosomes, fractions 4–7 contain light polysomes, and fractions 8–12 contain heavy polysomes. The data are presented as the mean ratio of VEGF to β-actin mRNA (± SD) obtained from triplicate samples. Similar results were obtained from three independent experiments.
Figure 4.
The α6β4 integrin stimulates the phosphorylation of Akt, 4E-BP1, and p70 S6K. (A) MDA-MB-435 parental cells, mock transfectants, and β4 transfectants were maintained in medium containing low serum (0.5% FBS) for 24 h. The phosphorylation status of 4E-BP1 on Ser 65 and S6K on Thr 389 was assessed in extracts from these cells using phosphospecific antibodies as described in the Materials and methods. In addition, the total amount of 4E-BP1 and p70S6K in these extracts was assessed by immunoblotting. (B) The MDA-MB-435/β4 cells were transiently transfected with either an eIF-4E sense (S) or antisense (AS) oligonucleotide, or a full-length eIF-4E cDNA (4E). Extracts of these cells containing equivalent amounts of protein were analyzed for their relative expression of VEGF and actin by immunoblotting. (C) MDA-MB-435 mock (clone 6D7) and β4 (clone 3A7) transfectants were maintained in low serum (0.5% FBS) medium for 24 h. These cells were detached with trypsin and incubated with integrin-specific antibodies (α6 integrin, 2B7; α6 integrin, GOH3; α5 integrin, Sam1; β4 integrin, A9) or IgG for 30 min as described in the legend to Fig. 2. The phosphorylation status of 4E-BP1 (Ser 65), S6K (Thr 389), and Akt (Ser 473) was assessed in extracts from these cells using phosphospecific antibodies. Similar results were observed in four independent experiments.
Figure 5.
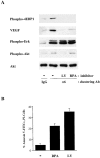
Stimulation of 4E-BP1 phosphorylation, VEGF expression, and survival by the α6β4 integrin requires PI-3K and mTOR. (A) MDA-MB-435 β4 transfectants (clone 3A7) were incubated with either DMSO (−), the PI-3K inhibitor LY 294002 (10 μM) (LY), or the mTOR-specific inhibitor rapamycin (50nM) (RPA) for 30 min and then incubated with either IgG or the α6 integrin antibody 2B7 as described in the legend to Fig. 2. Extracts of these cells were immunoblotted for phospho–4E-BP1 (Ser65), VEGF, phospho-Erk (recognizing phosphorylated isoforms of ERK1 and ERK2), phospho-Akt (Ser 473), and total Akt. Similar data were obtained in three experiments. (B) MDA-MB-435 β4 transfectants (clone 3A7) were maintained at low serum (0.5%) medium for 24 h in the presence of either rapamycin (50nM) (RPA), LY 294002 (10 μM) (LY), or DMSO (−). Apoptosis was assayed as described in the Materials and methods and is reported as the percentage of annexin V–FITC- positive, PI-negative cells. The data shown are mean values (± SD) of a representative experiment performed in triplicate.
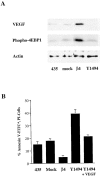
Figure 6.
Y1494 in the β4 cytoplasmic domain is required for α6β4 stimulation of 4E-BP1 phosphorylation, VEGF expression, and survival. (A) MDA-MB-435 parental cells (435), mock transfectants (clone 6D7), wild-type β4 transfectants (clone 3A7), and Y1494F mutant transfectants (clone E1h) were maintained in low serum (0.5% FBS) for 24 h. Extracts from these cells were analyzed by immunoblotting to assess the relative expression of VEGF and 4E-BP1 phosphorylation. The relative amount of actin was also determined as a control for protein loading. Similar results were obtained in three experiments. (B) Aliquots of the same cell populations described in A were assayed for the level of apoptosis after a 24-h incubation in low serum (0.5% FBS) medium. Apoptosis was assayed as described in the Materials and methods and is reported as the percentage of annexin V–FITC-positive, PI-negative cells. The data shown are mean values (± SD) of three experiments performed in triplicate.
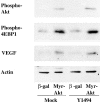
Figure 7.
Expression of a constitutively active Akt construct mimics the effects of α6β4 integrin expression and signaling. MDA-MB-435 mock transfectants (clone 6D7) and Y1494F mutant transfectants (clone E1h) were infected with adenoviruses that expressed either β-galactosidase or Myr-Akt as described in the Materials and methods. Subsequently, the cells were incubated in low serum (0.5% FBS) medium for 24 h. Extracts of these cells were immunoblotted to assess the relative phosphorylation of Akt and 4E-BP1, as well as total expression of VEGF and actin.
Figure 8.
α6β4 regulates 4E-BP1 phosphorylation, VEGF expression, and survival in carcinoma cells that express this integrin endogenously. (A) Parental MDA-MB-231 cells and cells transfected with antisense or sense VEGF oligonucleotides were maintained in low serum (0.5% FBS) medium for 24 h. Apoptosis was assayed as described in the Materials and methods and is reported as the percentage of annexin V–FITC-positive, PI-negative cells. The data shown are mean values (± SD) of two separate experiments performed in triplicate. (B) MDA-MB-231 cells were left untreated (UNT) or were transfected with either an RNAi specific for the β4 integrin (si-β4) or the corresponding inverted sequence (si-inv). After 72 h, the cells were placed in medium containing low serum (0.5% FBS) for an additional 24 h and then extracted. Extracts of these cells were immunoblotted as described in the legend to Fig. 4 to assess expression of β4 integrin, VEGF, and actin, as well as the phosphorylation of 4E-BP1. Similar results were observed in three independent trials. (C) Apoptosis was assessed in the same populations of cells and is reported as the percentage of annexin V–FITC-positive, PI-negative cells. The data shown are mean values (± SD) of three independent experiments performed in triplicate. (D) MDA-MB-231 cells were maintained in low serum (0.5% FBS) medium for 24 h and harvested by trypsin treatment. The suspended cells were incubated with integrin-specific antibodies (β4 integrin, A9; α6 integrin, 2B7; α5 integrin, Sam1) or IgG for 30 min in suspension and allowed to adhere on anti-IgG–coated plates for 30 min. Extracts of these cells were immunoblotted with phosphospecific antibodies to assess the relative phosphorylation of Akt and 4E-BP1, as well as with antibodies specific for VEGF and actin. Similar results were obtained in five experiments.
References
- Akiri, G., D. Nahari, Y. Finkelstein, S.Y. Le, O. Elroy-Stein, and B.Z. Levi. 1998. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 17:227–236. - PubMed
- Bachelder, R.E., A. Marchetti, R. Falcioni, S. Soddu, and A.M. Mercurio. 1999. a. Activation of p53 function in carcinoma cells by the alpha6beta4 integrin. J. Biol. Chem. 274:20733–20737. - PubMed
- Bachelder, R.E., A. Crago, J. Chung, M.A. Wendt, L.M. Shaw, G. Robinson, and A.M. Mercurio. 2001. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 61:5736–5740. - PubMed
- Berkel, H.J., E.A. Turbat-Herrera, R. Shi, and A. de Benedetti. 2001. Expression of the translation initiation factor eIF4E in the polyp-cancer sequence in the colon. Cancer Epidemiol. Biomarkers Prev. 10:663–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA81325/CA/NCI NIH HHS/United States
- CA81697/CA/NCI NIH HHS/United States
- CA80789/CA/NCI NIH HHS/United States
- R01 CA089209/CA/NCI NIH HHS/United States
- CA89209/CA/NCI NIH HHS/United States
- F32 CA081697/CA/NCI NIH HHS/United States
- R01 CA080789/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous