Characterization of the expression of MHC proteins in human embryonic stem cells - PubMed (original) (raw)
Characterization of the expression of MHC proteins in human embryonic stem cells
Micha Drukker et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Human embryonic stem (ES) cells are pluripotent cells that may be used in transplantation medicine. These cells can be induced to differentiate into cells from the three embryonic germ layers both in vivo and in vitro. To determine whether human ES cells might be rejected after transplantation, we examined cell surface expression of the MHC proteins in these cells. Our results show very low expression levels of MHC class I (MHC-I) proteins on the surface of human ES cells that moderately increase on in vitro or in vivo differentiation. A dramatic induction of MHC-I proteins was observed when the cells were treated with IFN-gamma but not with IFN-alpha or -beta. However, all three IFNs induced expression of MHC-I proteins in differentiated human ES cells. MHC-II proteins and HLA-G were not expressed on the surface of undifferentiated or differentiated cells. Ligands for natural killer cell receptors were either absent or expressed in very low levels in human ES cells and in their differentiated derivatives. In accordance, natural killer cytotoxic assays demonstrated only limited lysis of both undifferentiated and differentiated cells. To initiate a histocompatibility databank of human ES cells, we have isotyped several of the published ES cell lines for their human leukocyte antigens. In conclusion, our results demonstrate that human ES cells can express high levels of MHC-I proteins and thus may be rejected on transplantation.
Figures
Figure 1
Expression of MHC proteins in undifferentiated and differentiated human ES cells. FACS analysis of MHC molecules: expression of MHC-I was assayed by use of BBM1 and the pan-anticlass I W6/32 Abs. Expression of MHC-II was assayed by an Ab directed against HLA-DP, -DQ, -DR, and expression of the nonclassical MHC-I HLA-G was assayed by an Ab. Cell types: ES (H9) and ES (H13), two undifferentiated human ES cell lines; EB, in vitro differentiated human ES cells; Teratomas, _in vivo-_differentiated human ES cells; HeLa, cervix epithelial cell line used as a control for high expression level of MHC-I; 721/HLA-G, human B cell line 721.221 expressing HLA-G, used as control for high expression level of HLA-G and MHC-II. Dashed lines represent background staining with secondary Ab alone, and solid lines indicate expression of specific antigens. Median fluorescence intensity staining is indicated in the top of each box. Two to five independent experiments were performed for each analysis.
Figure 2
Effects of IFNs on the expression of MHC-I and HLA-E in undifferentiated and differentiated human ES cells. Shown are expression levels of β2 microglobulin, HLA-I and -E proteins. IFN-α, -β, or -γ was added to the growth media of human ES cells and teratoma cells for 48 h as described in Materials and Methods. Control levels of antigen expression are shown (Left). Median fluorescence intensity staining of the antigen is indicated at the top of each box. Dashed lines represent background staining with secondary Ab alone, and solid lines indicate expression of specific antigens. Two to five independent experiments were performed for each analysis.
Figure 3
IFN-γ induction of MHC-I in human ES cells is dose and time dependent. (A) Dose response of MHC-I expression on human ES cells on IFN-γ treatment. (B) Time dependence of MHC-I expression in human ES cells. (C) Time dependence of MHC-I expression after withdrawal of IFN-γ from the media. The FITC-conjugated secondary Ab was used as a negative control. Shown are standard error bars of three to four independent experiments.
Figure 4
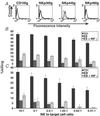
Human ES cell recognition by NK receptors and NK-mediated lysis. (A) FACS analysis for the expression of the NK-receptor specific ligands NKp44, NKp30, NKp46, and CD16 in human ES cells. Dashed lines represent background staining with secondary Ab alone, and solid lines indicate expression of specific antigens. Two independent experiments were performed for each analysis. Histogram representation of the cytotoxic effects of NK cells on naive and IFN-γ- (IFN-γ, 25 ng/ml, 48 h) treated human ES cells (B) and human EB cells (C). (B, C). The various NK-to-target cell ratios used are indicated. Shown are standard error bars of three experiments.
Similar articles
- Expression of immunoglobulin superfamily cell adhesion molecules on murine embryonic stem cells.
Tian L, Catt JW, O'Neill C, King NJ. Tian L, et al. Biol Reprod. 1997 Sep;57(3):561-8. doi: 10.1095/biolreprod57.3.561. Biol Reprod. 1997. PMID: 9282991 - Expression and regulation of major histocompatibility complex on neural stem cells and their lineages.
Yin L, Fu SL, Shi GY, Li Y, Jin JQ, Ma ZW, Lu PH. Yin L, et al. Stem Cells Dev. 2008 Feb;17(1):53-65. doi: 10.1089/scd.2007.0063. Stem Cells Dev. 2008. PMID: 18230026 - Variation in MHC expression between undifferentiated mouse ES cells and ES cell-derived insulin-producing cell clusters.
Boyd AS, Wood KJ. Boyd AS, et al. Transplantation. 2009 May 15;87(9):1300-4. doi: 10.1097/TP.0b013e3181a19421. Transplantation. 2009. PMID: 19424028 Free PMC article. - Human chromosome 16 encodes a factor involved in induction of class II major histocompatibility antigens by interferon gamma.
Bono MR, Alcaïde-Loridan C, Couillin P, Letouzé B, Grisard MC, Jouin H, Fellous M. Bono MR, et al. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6077-81. doi: 10.1073/pnas.88.14.6077. Proc Natl Acad Sci U S A. 1991. PMID: 1906174 Free PMC article. - Immunity of embryonic stem cell-derived hematopoietic progenitor cells.
Zavazava N. Zavazava N. Semin Immunopathol. 2011 Nov;33(6):613-7. doi: 10.1007/s00281-011-0273-9. Epub 2011 May 7. Semin Immunopathol. 2011. PMID: 21547436 Review.
Cited by
- Natural killer cell-activating receptor NKG2D mediates innate immune targeting of allogeneic neural progenitor cell grafts.
Phillips LK, Gould EA, Babu H, Krams SM, Palmer TD, Martinez OM. Phillips LK, et al. Stem Cells. 2013 Sep;31(9):1829-39. doi: 10.1002/stem.1422. Stem Cells. 2013. PMID: 23733329 Free PMC article. - Separate developmental programs for HLA-A and -B cell surface expression during differentiation from embryonic stem cells to lymphocytes, adipocytes and osteoblasts.
Sabir HJ, Nehlin JO, Qanie D, Harkness L, Prokhorova TA, Blagoev B, Kassem M, Isa A, Barington T. Sabir HJ, et al. PLoS One. 2013;8(1):e54366. doi: 10.1371/journal.pone.0054366. Epub 2013 Jan 18. PLoS One. 2013. PMID: 23349864 Free PMC article. - Immune Editing: Overcoming Immune Barriers in Stem Cell Transplantation.
Meissner TB, Schulze HS, Dale SM. Meissner TB, et al. Curr Stem Cell Rep. 2022;8(4):206-218. doi: 10.1007/s40778-022-00221-0. Epub 2022 Nov 8. Curr Stem Cell Rep. 2022. PMID: 36406259 Free PMC article. Review. - Heart repair and stem cells.
van Laake LW, Hassink R, Doevendans PA, Mummery C. van Laake LW, et al. J Physiol. 2006 Dec 1;577(Pt 2):467-78. doi: 10.1113/jphysiol.2006.115816. Epub 2006 Sep 28. J Physiol. 2006. PMID: 17008381 Free PMC article. Review. - Human embryonic stem cells: potential tool for achieving immunotolerance?
Menendez P, Bueno C, Wang L, Bhatia M. Menendez P, et al. Stem Cell Rev. 2005;1(2):151-8. doi: 10.1385/SCR:1:2:151. Stem Cell Rev. 2005. PMID: 17142850 Review.
References
- Thomson J A, Itskovitz-Eldor J, Shapiro S S, Waknitz M A, Swiergiel J J, Marshall V S, Jones J M. Science. 1998;282:1145–1147. - PubMed
- Reubinoff B E, Pera M F, Fong C Y, Trounson A, Bongso A. Nat Biotechnol. 2000;18:399–404. - PubMed
- Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein R S, Benvenisty N. Brain Res. 2001;913:201–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials