The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors - PubMed (original) (raw)
The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors
Yibing Yan et al. J Cell Biol. 2002.
Abstract
Communication between different signaling pathways enables cells to coordinate the responses to diverse environmental signals. Activation of the transmembrane growth factor precursors plays a critical role in this communication and often involves metalloprotease-mediated proteolysis. Stimulation of G protein-coupled receptors (GPCR) transactivates the EGF receptors (EGFRs), which occurs via a metalloprotease-dependent cleavage of heparin-binding EGF (HB-EGF). However, the metalloprotease mediating the transactivation remains elusive. We show that the integral membrane metalloprotease Kuzbanian (KUZ; ADAM10), which controls Notch signaling in Drosophila, stimulates GPCR transactivation of EGFR. Upon stimulation of the bombesin receptors, KUZ increases the docking and activation of adaptors Src homology 2 domain-containing protein and Gab1 on the EGFR, and activation of Ras and Erk. In contrast, transfection of a protease domain-deleted KUZ, or blocking endogenous KUZ by morpholino antisense oligonucleotides, suppresses the transactivation. The effect of KUZ on shedding of HB-EGF and consequent transactivation of the EGFR depends on its metalloprotease activity. GPCR activation enhances the association of KUZ and its substrate HB-EGF with tetraspanin CD9. Thus, KUZ regulates the relay between the GPCR and EGFR signaling pathways.
Figures
Figure 1.
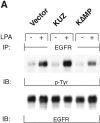
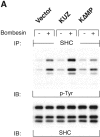
KUZ mediates GPCR transactivation of EGFR in COS7 cells. (A and B) KUZ, but not KΔMP, stimulates the LPA- and bombesin-induced EGFR phosphorylation. EGFR activation was detected in precipitated EGFR with antiphosphotyrosine antibody 4G10 and the amount of EGFR was detected with goat anti-EGFR antibody in the same blot. (C) Increase of EGFR phosphorylation induced by bombesin in the presence of exogenous KUZ or KΔMP. Phosphorylation was quantified with NIH Image 1.62 (n = 6 sets of experiments). (D) Bombesin-induced EGFR phosphorylation depends on HB-EGF and EGFR kinase. COS7 cells were pretreated for 20 min with CRM197 (lanes 3 and 4) or specific EGFR kinase inhibitor AG1487 (lanes 5 and 6) before treatment with bombesin. (E–G) KUZ is more effective than ADAM17 (TACE) or ADAM15 in mediating bombesin-induced EGFR transactivation. Transactivation of EGFR (E–G) and activation of the downstream adaptor SHC (E) are shown in cells transfected with equal amounts of plasmids containing (E) wild-type TACE and KUZ or (F) COOH-terminal Flag-tagged TACE, ADAM15, and KUZ. The amount of ADAMs expressed is shown in an immunoblot of cell lysates with anti-Flag antibody M2. Results (n = 3 sets of experiments) are quantified in G.
Figure 1.
KUZ mediates GPCR transactivation of EGFR in COS7 cells. (A and B) KUZ, but not KΔMP, stimulates the LPA- and bombesin-induced EGFR phosphorylation. EGFR activation was detected in precipitated EGFR with antiphosphotyrosine antibody 4G10 and the amount of EGFR was detected with goat anti-EGFR antibody in the same blot. (C) Increase of EGFR phosphorylation induced by bombesin in the presence of exogenous KUZ or KΔMP. Phosphorylation was quantified with NIH Image 1.62 (n = 6 sets of experiments). (D) Bombesin-induced EGFR phosphorylation depends on HB-EGF and EGFR kinase. COS7 cells were pretreated for 20 min with CRM197 (lanes 3 and 4) or specific EGFR kinase inhibitor AG1487 (lanes 5 and 6) before treatment with bombesin. (E–G) KUZ is more effective than ADAM17 (TACE) or ADAM15 in mediating bombesin-induced EGFR transactivation. Transactivation of EGFR (E–G) and activation of the downstream adaptor SHC (E) are shown in cells transfected with equal amounts of plasmids containing (E) wild-type TACE and KUZ or (F) COOH-terminal Flag-tagged TACE, ADAM15, and KUZ. The amount of ADAMs expressed is shown in an immunoblot of cell lysates with anti-Flag antibody M2. Results (n = 3 sets of experiments) are quantified in G.
Figure 1.
KUZ mediates GPCR transactivation of EGFR in COS7 cells. (A and B) KUZ, but not KΔMP, stimulates the LPA- and bombesin-induced EGFR phosphorylation. EGFR activation was detected in precipitated EGFR with antiphosphotyrosine antibody 4G10 and the amount of EGFR was detected with goat anti-EGFR antibody in the same blot. (C) Increase of EGFR phosphorylation induced by bombesin in the presence of exogenous KUZ or KΔMP. Phosphorylation was quantified with NIH Image 1.62 (n = 6 sets of experiments). (D) Bombesin-induced EGFR phosphorylation depends on HB-EGF and EGFR kinase. COS7 cells were pretreated for 20 min with CRM197 (lanes 3 and 4) or specific EGFR kinase inhibitor AG1487 (lanes 5 and 6) before treatment with bombesin. (E–G) KUZ is more effective than ADAM17 (TACE) or ADAM15 in mediating bombesin-induced EGFR transactivation. Transactivation of EGFR (E–G) and activation of the downstream adaptor SHC (E) are shown in cells transfected with equal amounts of plasmids containing (E) wild-type TACE and KUZ or (F) COOH-terminal Flag-tagged TACE, ADAM15, and KUZ. The amount of ADAMs expressed is shown in an immunoblot of cell lysates with anti-Flag antibody M2. Results (n = 3 sets of experiments) are quantified in G.
Figure 1.
KUZ mediates GPCR transactivation of EGFR in COS7 cells. (A and B) KUZ, but not KΔMP, stimulates the LPA- and bombesin-induced EGFR phosphorylation. EGFR activation was detected in precipitated EGFR with antiphosphotyrosine antibody 4G10 and the amount of EGFR was detected with goat anti-EGFR antibody in the same blot. (C) Increase of EGFR phosphorylation induced by bombesin in the presence of exogenous KUZ or KΔMP. Phosphorylation was quantified with NIH Image 1.62 (n = 6 sets of experiments). (D) Bombesin-induced EGFR phosphorylation depends on HB-EGF and EGFR kinase. COS7 cells were pretreated for 20 min with CRM197 (lanes 3 and 4) or specific EGFR kinase inhibitor AG1487 (lanes 5 and 6) before treatment with bombesin. (E–G) KUZ is more effective than ADAM17 (TACE) or ADAM15 in mediating bombesin-induced EGFR transactivation. Transactivation of EGFR (E–G) and activation of the downstream adaptor SHC (E) are shown in cells transfected with equal amounts of plasmids containing (E) wild-type TACE and KUZ or (F) COOH-terminal Flag-tagged TACE, ADAM15, and KUZ. The amount of ADAMs expressed is shown in an immunoblot of cell lysates with anti-Flag antibody M2. Results (n = 3 sets of experiments) are quantified in G.
Figure 1.
KUZ mediates GPCR transactivation of EGFR in COS7 cells. (A and B) KUZ, but not KΔMP, stimulates the LPA- and bombesin-induced EGFR phosphorylation. EGFR activation was detected in precipitated EGFR with antiphosphotyrosine antibody 4G10 and the amount of EGFR was detected with goat anti-EGFR antibody in the same blot. (C) Increase of EGFR phosphorylation induced by bombesin in the presence of exogenous KUZ or KΔMP. Phosphorylation was quantified with NIH Image 1.62 (n = 6 sets of experiments). (D) Bombesin-induced EGFR phosphorylation depends on HB-EGF and EGFR kinase. COS7 cells were pretreated for 20 min with CRM197 (lanes 3 and 4) or specific EGFR kinase inhibitor AG1487 (lanes 5 and 6) before treatment with bombesin. (E–G) KUZ is more effective than ADAM17 (TACE) or ADAM15 in mediating bombesin-induced EGFR transactivation. Transactivation of EGFR (E–G) and activation of the downstream adaptor SHC (E) are shown in cells transfected with equal amounts of plasmids containing (E) wild-type TACE and KUZ or (F) COOH-terminal Flag-tagged TACE, ADAM15, and KUZ. The amount of ADAMs expressed is shown in an immunoblot of cell lysates with anti-Flag antibody M2. Results (n = 3 sets of experiments) are quantified in G.
Figure 1.
KUZ mediates GPCR transactivation of EGFR in COS7 cells. (A and B) KUZ, but not KΔMP, stimulates the LPA- and bombesin-induced EGFR phosphorylation. EGFR activation was detected in precipitated EGFR with antiphosphotyrosine antibody 4G10 and the amount of EGFR was detected with goat anti-EGFR antibody in the same blot. (C) Increase of EGFR phosphorylation induced by bombesin in the presence of exogenous KUZ or KΔMP. Phosphorylation was quantified with NIH Image 1.62 (n = 6 sets of experiments). (D) Bombesin-induced EGFR phosphorylation depends on HB-EGF and EGFR kinase. COS7 cells were pretreated for 20 min with CRM197 (lanes 3 and 4) or specific EGFR kinase inhibitor AG1487 (lanes 5 and 6) before treatment with bombesin. (E–G) KUZ is more effective than ADAM17 (TACE) or ADAM15 in mediating bombesin-induced EGFR transactivation. Transactivation of EGFR (E–G) and activation of the downstream adaptor SHC (E) are shown in cells transfected with equal amounts of plasmids containing (E) wild-type TACE and KUZ or (F) COOH-terminal Flag-tagged TACE, ADAM15, and KUZ. The amount of ADAMs expressed is shown in an immunoblot of cell lysates with anti-Flag antibody M2. Results (n = 3 sets of experiments) are quantified in G.
Figure 1.
KUZ mediates GPCR transactivation of EGFR in COS7 cells. (A and B) KUZ, but not KΔMP, stimulates the LPA- and bombesin-induced EGFR phosphorylation. EGFR activation was detected in precipitated EGFR with antiphosphotyrosine antibody 4G10 and the amount of EGFR was detected with goat anti-EGFR antibody in the same blot. (C) Increase of EGFR phosphorylation induced by bombesin in the presence of exogenous KUZ or KΔMP. Phosphorylation was quantified with NIH Image 1.62 (n = 6 sets of experiments). (D) Bombesin-induced EGFR phosphorylation depends on HB-EGF and EGFR kinase. COS7 cells were pretreated for 20 min with CRM197 (lanes 3 and 4) or specific EGFR kinase inhibitor AG1487 (lanes 5 and 6) before treatment with bombesin. (E–G) KUZ is more effective than ADAM17 (TACE) or ADAM15 in mediating bombesin-induced EGFR transactivation. Transactivation of EGFR (E–G) and activation of the downstream adaptor SHC (E) are shown in cells transfected with equal amounts of plasmids containing (E) wild-type TACE and KUZ or (F) COOH-terminal Flag-tagged TACE, ADAM15, and KUZ. The amount of ADAMs expressed is shown in an immunoblot of cell lysates with anti-Flag antibody M2. Results (n = 3 sets of experiments) are quantified in G.
Figure 2.
KUZ stimulates, and blocking endogenous KUZ inhibits, the transactivation of signaling pathways downstream of EGFR. (A) Activation of all three forms of SHC and (B) Gab1 phosphorylation by GPCR are stimulated by transfecting KUZ, but not protease-deleted KΔMP, into COS7 cells. (C) KUZ elevates GPCR-induced Ras activation in COS7 cells. The upper band, nonspecifically reactive to the anti-Ras antibody, is shown as the loading control. (D) Bombesin induces Erk1/2 activation in PC3 cells, which is partly due to the transactivation of EGFR and depends on HB-EGF release. PC3 cells were treated with CRM197 or AG1487 and then stimulated with bombesin. (E) Transfecting KΔMP suppresses Erk1/2 activation in PC3 cells. Active Erk1/2 was detected in bombesin-treated or untreated PC3 cells transfected with vector or KΔMP with anti–phospho-Erk1/2 antibody. (F) Bombesin-induced Erk1/2 activation can be inhibited by an antisense morpholino oligonucleotide against ADAM10. Erk1/2 phosphorylation was detected in lysates of PC3 treated with the morpholino anti-ADAM10 oligo (Anti-KUZ) and control oligo with the same base composition but in reverse order (Reverse-KUZ). The endogenous ADAM10 in antisense oligo–treated cells was detected with a polyclonal antibody to ADAM10 (KUZ).
Figure 2.
KUZ stimulates, and blocking endogenous KUZ inhibits, the transactivation of signaling pathways downstream of EGFR. (A) Activation of all three forms of SHC and (B) Gab1 phosphorylation by GPCR are stimulated by transfecting KUZ, but not protease-deleted KΔMP, into COS7 cells. (C) KUZ elevates GPCR-induced Ras activation in COS7 cells. The upper band, nonspecifically reactive to the anti-Ras antibody, is shown as the loading control. (D) Bombesin induces Erk1/2 activation in PC3 cells, which is partly due to the transactivation of EGFR and depends on HB-EGF release. PC3 cells were treated with CRM197 or AG1487 and then stimulated with bombesin. (E) Transfecting KΔMP suppresses Erk1/2 activation in PC3 cells. Active Erk1/2 was detected in bombesin-treated or untreated PC3 cells transfected with vector or KΔMP with anti–phospho-Erk1/2 antibody. (F) Bombesin-induced Erk1/2 activation can be inhibited by an antisense morpholino oligonucleotide against ADAM10. Erk1/2 phosphorylation was detected in lysates of PC3 treated with the morpholino anti-ADAM10 oligo (Anti-KUZ) and control oligo with the same base composition but in reverse order (Reverse-KUZ). The endogenous ADAM10 in antisense oligo–treated cells was detected with a polyclonal antibody to ADAM10 (KUZ).
Figure 2.
KUZ stimulates, and blocking endogenous KUZ inhibits, the transactivation of signaling pathways downstream of EGFR. (A) Activation of all three forms of SHC and (B) Gab1 phosphorylation by GPCR are stimulated by transfecting KUZ, but not protease-deleted KΔMP, into COS7 cells. (C) KUZ elevates GPCR-induced Ras activation in COS7 cells. The upper band, nonspecifically reactive to the anti-Ras antibody, is shown as the loading control. (D) Bombesin induces Erk1/2 activation in PC3 cells, which is partly due to the transactivation of EGFR and depends on HB-EGF release. PC3 cells were treated with CRM197 or AG1487 and then stimulated with bombesin. (E) Transfecting KΔMP suppresses Erk1/2 activation in PC3 cells. Active Erk1/2 was detected in bombesin-treated or untreated PC3 cells transfected with vector or KΔMP with anti–phospho-Erk1/2 antibody. (F) Bombesin-induced Erk1/2 activation can be inhibited by an antisense morpholino oligonucleotide against ADAM10. Erk1/2 phosphorylation was detected in lysates of PC3 treated with the morpholino anti-ADAM10 oligo (Anti-KUZ) and control oligo with the same base composition but in reverse order (Reverse-KUZ). The endogenous ADAM10 in antisense oligo–treated cells was detected with a polyclonal antibody to ADAM10 (KUZ).
Figure 2.
KUZ stimulates, and blocking endogenous KUZ inhibits, the transactivation of signaling pathways downstream of EGFR. (A) Activation of all three forms of SHC and (B) Gab1 phosphorylation by GPCR are stimulated by transfecting KUZ, but not protease-deleted KΔMP, into COS7 cells. (C) KUZ elevates GPCR-induced Ras activation in COS7 cells. The upper band, nonspecifically reactive to the anti-Ras antibody, is shown as the loading control. (D) Bombesin induces Erk1/2 activation in PC3 cells, which is partly due to the transactivation of EGFR and depends on HB-EGF release. PC3 cells were treated with CRM197 or AG1487 and then stimulated with bombesin. (E) Transfecting KΔMP suppresses Erk1/2 activation in PC3 cells. Active Erk1/2 was detected in bombesin-treated or untreated PC3 cells transfected with vector or KΔMP with anti–phospho-Erk1/2 antibody. (F) Bombesin-induced Erk1/2 activation can be inhibited by an antisense morpholino oligonucleotide against ADAM10. Erk1/2 phosphorylation was detected in lysates of PC3 treated with the morpholino anti-ADAM10 oligo (Anti-KUZ) and control oligo with the same base composition but in reverse order (Reverse-KUZ). The endogenous ADAM10 in antisense oligo–treated cells was detected with a polyclonal antibody to ADAM10 (KUZ).
Figure 2.
KUZ stimulates, and blocking endogenous KUZ inhibits, the transactivation of signaling pathways downstream of EGFR. (A) Activation of all three forms of SHC and (B) Gab1 phosphorylation by GPCR are stimulated by transfecting KUZ, but not protease-deleted KΔMP, into COS7 cells. (C) KUZ elevates GPCR-induced Ras activation in COS7 cells. The upper band, nonspecifically reactive to the anti-Ras antibody, is shown as the loading control. (D) Bombesin induces Erk1/2 activation in PC3 cells, which is partly due to the transactivation of EGFR and depends on HB-EGF release. PC3 cells were treated with CRM197 or AG1487 and then stimulated with bombesin. (E) Transfecting KΔMP suppresses Erk1/2 activation in PC3 cells. Active Erk1/2 was detected in bombesin-treated or untreated PC3 cells transfected with vector or KΔMP with anti–phospho-Erk1/2 antibody. (F) Bombesin-induced Erk1/2 activation can be inhibited by an antisense morpholino oligonucleotide against ADAM10. Erk1/2 phosphorylation was detected in lysates of PC3 treated with the morpholino anti-ADAM10 oligo (Anti-KUZ) and control oligo with the same base composition but in reverse order (Reverse-KUZ). The endogenous ADAM10 in antisense oligo–treated cells was detected with a polyclonal antibody to ADAM10 (KUZ).
Figure 2.
KUZ stimulates, and blocking endogenous KUZ inhibits, the transactivation of signaling pathways downstream of EGFR. (A) Activation of all three forms of SHC and (B) Gab1 phosphorylation by GPCR are stimulated by transfecting KUZ, but not protease-deleted KΔMP, into COS7 cells. (C) KUZ elevates GPCR-induced Ras activation in COS7 cells. The upper band, nonspecifically reactive to the anti-Ras antibody, is shown as the loading control. (D) Bombesin induces Erk1/2 activation in PC3 cells, which is partly due to the transactivation of EGFR and depends on HB-EGF release. PC3 cells were treated with CRM197 or AG1487 and then stimulated with bombesin. (E) Transfecting KΔMP suppresses Erk1/2 activation in PC3 cells. Active Erk1/2 was detected in bombesin-treated or untreated PC3 cells transfected with vector or KΔMP with anti–phospho-Erk1/2 antibody. (F) Bombesin-induced Erk1/2 activation can be inhibited by an antisense morpholino oligonucleotide against ADAM10. Erk1/2 phosphorylation was detected in lysates of PC3 treated with the morpholino anti-ADAM10 oligo (Anti-KUZ) and control oligo with the same base composition but in reverse order (Reverse-KUZ). The endogenous ADAM10 in antisense oligo–treated cells was detected with a polyclonal antibody to ADAM10 (KUZ).
Figure 3.
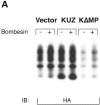
Metalloprotease activity of KUZ is responsible for the GPCR transactivation EGFR signaling pathway in COS7 cells. (A) Transfection of wild-type KUZ, but not KΔMP, stimulates the release of soluble HB-EGF into the medium. The HA-tagged HB-EGF in the medium of transfected cells was analyzed by collecting the heparin-binding proteins and blotting with the anti-HA antibody 12CA5. (B) Metalloprotease inhibitor TAPI blocks bombesin-induced transactivation of EGFR and SHC. Cells were preincubated with either TAPI or solvent DMSO before stimulation with bombesin. The immunoprecipitated EGFR or SHC was blotted with antiphosphotyrosine antibody 4G10 to reveal the activated EGFR and SHC. (C) Catalytically inactive KUZ (K[E-A]) does not support the EGFR and SHC transactivation. The total EGFR and SHC was equivalent in each lane (not depicted).
Figure 3.
Metalloprotease activity of KUZ is responsible for the GPCR transactivation EGFR signaling pathway in COS7 cells. (A) Transfection of wild-type KUZ, but not KΔMP, stimulates the release of soluble HB-EGF into the medium. The HA-tagged HB-EGF in the medium of transfected cells was analyzed by collecting the heparin-binding proteins and blotting with the anti-HA antibody 12CA5. (B) Metalloprotease inhibitor TAPI blocks bombesin-induced transactivation of EGFR and SHC. Cells were preincubated with either TAPI or solvent DMSO before stimulation with bombesin. The immunoprecipitated EGFR or SHC was blotted with antiphosphotyrosine antibody 4G10 to reveal the activated EGFR and SHC. (C) Catalytically inactive KUZ (K[E-A]) does not support the EGFR and SHC transactivation. The total EGFR and SHC was equivalent in each lane (not depicted).
Figure 3.
Metalloprotease activity of KUZ is responsible for the GPCR transactivation EGFR signaling pathway in COS7 cells. (A) Transfection of wild-type KUZ, but not KΔMP, stimulates the release of soluble HB-EGF into the medium. The HA-tagged HB-EGF in the medium of transfected cells was analyzed by collecting the heparin-binding proteins and blotting with the anti-HA antibody 12CA5. (B) Metalloprotease inhibitor TAPI blocks bombesin-induced transactivation of EGFR and SHC. Cells were preincubated with either TAPI or solvent DMSO before stimulation with bombesin. The immunoprecipitated EGFR or SHC was blotted with antiphosphotyrosine antibody 4G10 to reveal the activated EGFR and SHC. (C) Catalytically inactive KUZ (K[E-A]) does not support the EGFR and SHC transactivation. The total EGFR and SHC was equivalent in each lane (not depicted).
Figure 4.
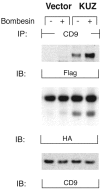
GPCRs regulate the formation of a complex of CD9 with KUZ and HB-EGF. COS7 cells were transfected with Flag-tagged KUZ and HA-tagged HB-EGF. CD9 was precipitated from CHAPS lysates of control or bombesin-treated cells with a polyclonal anti-CD9 antibody. Proteins from the immunoprecipitation were blotted with monoclonal antibodies against Flag epitope, HA epitope, and CD9, respectively.
Similar articles
- EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF.
Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. Prenzel N, et al. Nature. 1999 Dec 23-30;402(6764):884-8. doi: 10.1038/47260. Nature. 1999. PMID: 10622253 - G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor.
Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, Eckhart AD, Dempsey PJ, Eguchi S. Mifune M, et al. J Biol Chem. 2005 Jul 15;280(28):26592-9. doi: 10.1074/jbc.M502906200. Epub 2005 May 19. J Biol Chem. 2005. PMID: 15905175 - Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding.
Pierce KL, Tohgo A, Ahn S, Field ME, Luttrell LM, Lefkowitz RJ. Pierce KL, et al. J Biol Chem. 2001 Jun 22;276(25):23155-60. doi: 10.1074/jbc.M101303200. Epub 2001 Apr 4. J Biol Chem. 2001. PMID: 11290747 - Developmental signaling: notch signals Kuz it's cleaved.
Nye JS. Nye JS. Curr Biol. 1997 Nov 1;7(11):R716-20. doi: 10.1016/s0960-9822(06)00364-2. Curr Biol. 1997. PMID: 9382794 Review. - Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands.
Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Higashiyama S, et al. Cancer Sci. 2008 Feb;99(2):214-20. doi: 10.1111/j.1349-7006.2007.00676.x. Cancer Sci. 2008. PMID: 18271917 Free PMC article. Review.
Cited by
- Identification of novel genes that regulate androgen receptor signaling and growth of androgen-deprived prostate cancer cells.
Levina E, Ji H, Chen M, Baig M, Oliver D, Ohouo P, Lim CU, Schools G, Carmack S, Ding Y, Broude EV, Roninson IB, Buttyan R, Shtutman M. Levina E, et al. Oncotarget. 2015 May 30;6(15):13088-104. doi: 10.18632/oncotarget.3743. Oncotarget. 2015. PMID: 26036626 Free PMC article. - Epidermal growth factor receptor tyrosine kinase-mediated signalling contributes to diabetes-induced vascular dysfunction in the mesenteric bed.
Benter IF, Yousif MH, Griffiths SM, Benboubetra M, Akhtar S. Benter IF, et al. Br J Pharmacol. 2005 Jul;145(6):829-36. doi: 10.1038/sj.bjp.0706238. Br J Pharmacol. 2005. PMID: 15852031 Free PMC article. - Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling.
Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Jackson LF, et al. EMBO J. 2003 Jun 2;22(11):2704-16. doi: 10.1093/emboj/cdg264. EMBO J. 2003. PMID: 12773386 Free PMC article. - The association of the expression of miR-122-5p and its target ADAM10 with human breast cancer.
Ergün S, Ulasli M, Igci YZ, Igci M, Kırkbes S, Borazan E, Balik A, Yumrutaş Ö, Camci C, Cakmak EA, Arslan A, Oztuzcu S. Ergün S, et al. Mol Biol Rep. 2015 Feb;42(2):497-505. doi: 10.1007/s11033-014-3793-2. Epub 2014 Oct 16. Mol Biol Rep. 2015. PMID: 25318895
References
- Black, R.A., and J.M. White. 1998. ADAMs: focus on the protease domain. Curr. Opin. Cell Biol. 10:654–659. - PubMed
- Blobel, C.P. 2000. Remarkable roles of proteolysis on and beyond the cell surface. Curr. Opin. Cell Biol. 12:606–612. - PubMed
- Chen, M.S., K.S. Tung, S.A. Coonrod, Y. Takahashi, D. Bigler, A. Chang, Y. Yamashita, P.W. Kincade, J.C. Herr, and J.M. White. 1999. Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin α6β1: implications for murine fertilization. Proc. Natl. Acad. Sci. USA. 96:11830–11835. - PMC - PubMed
- Daub, H., F.U. Weiss, C. Wallasch, and A. Ullrich. 1996. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 379:557–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous