Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride - PubMed (original) (raw)
Case Reports
. 2002 Jul;110(2):259-69.
doi: 10.1172/JCI15311.
Affiliations
- PMID: 12122118
- PMCID: PMC151060
- DOI: 10.1172/JCI15311
Case Reports
Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride
Charles R Roe et al. J Clin Invest. 2002 Jul.
Abstract
The current dietary treatment of long-chain fatty acid oxidation defects (high carbohydrate with medium-even-chain triglycerides and reduced amounts of long-chain fats) fails, in many cases, to prevent cardiomyopathy, rhabdomyolysis, and muscle weakness. We hypothesized that the apparent defect in energy production results from a depletion of the catalytic intermediates of the citric acid cycle via leakage through cell membranes (cataplerosis). We further hypothesized that replacing dietary medium-even-chain fatty acids (precursors of acetyl-CoA) by medium-odd-chain fatty acids (precursors of acetyl-CoA and anaplerotic propionyl-CoA) would restore energy production and improve cardiac and skeletal muscle function. We fed subjects with long-chain defects a controlled diet in which the fat component was switched from medium-even-chain triglycerides to triheptanoin. In three patients with very-long-chain acyl-CoA dehydrogenase deficiency, this treatment led rapidly to clinical improvement that included the permanent disappearance of chronic cardiomyopathy, rhabdomyolysis, and muscle weakness (for more than 2 years in one child), and of rhabdomyolysis and weakness in the others. There was no evidence of propionyl overload in these patients. The treatment has been well tolerated for up to 26 months and opens new avenues for the management of patients with mitochondrial fat oxidation disorders.
Figures
Figure 1
Production of [3-2H3]propionylcarnitine from C7, C9, and C15 by cultured fibroblasts from the VLCAD patient (black bars) and 50 control subjects (white bars, mean ± SE).
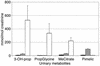
Figure 2
Urinary excretion of indices of propionyl overload (3-hydroxypropionate [3-OH-prop], propionylglycine [PropGlycine], methylcitrate [MeCitrate]) and pimelic in urine samples from 50 normal subjects (black bars), the VLCAD patients (gray bars; ten samples collected over 12 months), and ten patients with propionic acidemia (white bars).
Similar articles
- Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: a double blinded, randomized controlled trial.
Gillingham MB, Heitner SB, Martin J, Rose S, Goldstein A, El-Gharbawy AH, Deward S, Lasarev MR, Pollaro J, DeLany JP, Burchill LJ, Goodpaster B, Shoemaker J, Matern D, Harding CO, Vockley J. Gillingham MB, et al. J Inherit Metab Dis. 2017 Nov;40(6):831-843. doi: 10.1007/s10545-017-0085-8. Epub 2017 Sep 4. J Inherit Metab Dis. 2017. PMID: 28871440 Free PMC article. Clinical Trial. - Cardiac tissue citric acid cycle intermediates in exercised very long-chain acyl-CoA dehydrogenase-deficient mice fed triheptanoin or medium-chain triglyceride.
Gaston G, Gangoiti JA, Winn S, Chan B, Barshop BA, Harding CO, Gillingham MB. Gaston G, et al. J Inherit Metab Dis. 2020 Nov;43(6):1232-1242. doi: 10.1002/jimd.12284. Epub 2020 Aug 4. J Inherit Metab Dis. 2020. PMID: 33448436 - De novo fatty acid biosynthesis and elongation in very long-chain acyl-CoA dehydrogenase-deficient mice supplemented with odd or even medium-chain fatty acids.
Tucci S, Behringer S, Spiekerkoetter U. Tucci S, et al. FEBS J. 2015 Nov;282(21):4242-53. doi: 10.1111/febs.13418. Epub 2015 Sep 11. FEBS J. 2015. PMID: 26284828 - Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment--A retrospective chart review.
Vockley J, Marsden D, McCracken E, DeWard S, Barone A, Hsu K, Kakkis E. Vockley J, et al. Mol Genet Metab. 2015 Sep-Oct;116(1-2):53-60. doi: 10.1016/j.ymgme.2015.06.006. Epub 2015 Jun 18. Mol Genet Metab. 2015. PMID: 26116311 Free PMC article. Review. - Energy exchangers with LCT as a precision method for diet control in LCHADD.
Mozrzymas R, Konikowska K, Regulska-Ilow B. Mozrzymas R, et al. Adv Clin Exp Med. 2017 May-Jun;26(3):515-525. doi: 10.17219/acem/62132. Adv Clin Exp Med. 2017. PMID: 28791828 Review.
Cited by
- Complex I assembly function and fatty acid oxidation enzyme activity of ACAD9 both contribute to disease severity in ACAD9 deficiency.
Schiff M, Haberberger B, Xia C, Mohsen AW, Goetzman ES, Wang Y, Uppala R, Zhang Y, Karunanidhi A, Prabhu D, Alharbi H, Prochownik EV, Haack T, Häberle J, Munnich A, Rötig A, Taylor RW, Nicholls RD, Kim JJ, Prokisch H, Vockley J. Schiff M, et al. Hum Mol Genet. 2015 Jun 1;24(11):3238-47. doi: 10.1093/hmg/ddv074. Epub 2015 Feb 26. Hum Mol Genet. 2015. PMID: 25721401 Free PMC article. - Clinical manifestations and management of fatty acid oxidation disorders.
Merritt JL 2nd, MacLeod E, Jurecka A, Hainline B. Merritt JL 2nd, et al. Rev Endocr Metab Disord. 2020 Dec;21(4):479-493. doi: 10.1007/s11154-020-09568-3. Rev Endocr Metab Disord. 2020. PMID: 32654032 Free PMC article. Review. - Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency.
Gillingham MB, Connor WE, Matern D, Rinaldo P, Burlingame T, Meeuws K, Harding CO. Gillingham MB, et al. Mol Genet Metab. 2003 Jun;79(2):114-23. doi: 10.1016/s1096-7192(03)00073-8. Mol Genet Metab. 2003. PMID: 12809642 Free PMC article. Clinical Trial. - Real-time microscopic assessment of fatty acid uptake kinetics in the human term placenta.
Kolahi KS, Valent AM, Thornburg KL. Kolahi KS, et al. Placenta. 2018 Dec;72-73:1-9. doi: 10.1016/j.placenta.2018.07.014. Epub 2018 Jul 31. Placenta. 2018. PMID: 30501875 Free PMC article. - Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: a double blinded, randomized controlled trial.
Gillingham MB, Heitner SB, Martin J, Rose S, Goldstein A, El-Gharbawy AH, Deward S, Lasarev MR, Pollaro J, DeLany JP, Burchill LJ, Goodpaster B, Shoemaker J, Matern D, Harding CO, Vockley J. Gillingham MB, et al. J Inherit Metab Dis. 2017 Nov;40(6):831-843. doi: 10.1007/s10545-017-0085-8. Epub 2017 Sep 4. J Inherit Metab Dis. 2017. PMID: 28871440 Free PMC article. Clinical Trial.
References
- Dimauro S, Dimauro PMM. Muscle carnitine palmitoyl transferase deficiency and myoglobinuria. Science. 1973;182:929–931. - PubMed
- Engel AG, Angelini C. Carnitine deficiency of human skeletal muscle associated with lipid storage myopathy: a new syndrome. Science. 1973;179:899–901. - PubMed
- Roe, C.R., and Ding, J.H. 2001. Mitochondrial fatty acid oxidation disorders. In The metabolic and molecular bases of inherited disease. 8th edition. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 2297–2326.
- Sweetman L. Newborn screening by tandem mass spectrometry: gaining experience. Clin Chem. 2001;47:1937–1938. - PubMed
- Saudubray JM, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22:488–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical