The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans - PubMed (original) (raw)
The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans
James F Morley et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Studies of the mutant gene in Huntington's disease, and for eight related neurodegenerative disorders, have identified polyglutamine (polyQ) expansions as a basis for cellular toxicity. This finding has led to a disease hypothesis that protein aggregation and cellular dysfunction can occur at a threshold of approximately 40 glutamine residues. Here, we test this hypothesis by expression of fluorescently tagged polyQ proteins (Q29, Q33, Q35, Q40, and Q44) in the body wall muscle cells of Caenorhabditis elegans and show that young adults exhibit a sharp boundary at 35-40 glutamines associated with the appearance of protein aggregates and loss of motility. Surprisingly, genetically identical animals expressing near-threshold polyQ repeats exhibited a high degree of variation in the appearance of protein aggregates and cellular toxicity that was dependent on repeat length and exacerbated during aging. The role of genetically determined aging pathways in the progression of age-dependent polyQ-mediated aggregation and cellular toxicity was tested by expressing Q82 in the background of age-1 mutant animals that exhibit an extended lifespan. We observed a dramatic delay of polyQ toxicity and appearance of protein aggregates. These data provide experimental support for the threshold hypothesis of polyQ-mediated toxicity in an experimental organism and emphasize the importance of the threshold as a point at which genetic modifiers and aging influence biochemical environment and protein homeostasis in the cell.
Figures
Fig 1.
Length-dependent aggregation of polyQ-YFP fusion proteins in C. elegans. Epifluoresence micrographs of 3- to 4-day-old C. elegans expressing different lengths of polyQ-YFP (Q0, Q19, Q29, Q33, Q35, Q40, Q44, Q64, Q82). (Bar = 0.1 mm.)
Fig 2.
Determination of polyQ-YFP solubility in living animals by using FRAP. (Left: A, D, G, J, M) Merged phase-contrast and fluorescence images (pseudocolored yellow). (Center: B, E, H, K, N) Fluorescence images of the same region before photobleaching (prebleach). Boxes indicate the area that was subjected to photobleaching. (Right: C, F, I, L, O) Fluorescence images of recovery at the indicated times after photobleaching. The earliest time point possible to assess recovery of the chimeric YFP signal was at 3 s. (Bars = 3 μm.)
Fig 3.
Expression of polyQ expansions in C. elegans muscle results in a motility defect that directly corresponds to aggregate formation. (A) Time-lapse micrographs illustrating tracks left by 5-day-old wild-type (N2) and Q82 animals 2 and 30 min after being placed at the position marked by the red arrow. (B) Quantitation of motility index for 4- to 5-day-old Q0, Q19, Q29, Q35, Q40, Q82, and unc-54(r293) animals. Data are mean ± SD for at least 50 animals of each type as a percentage of N2 motility. (C_–_E) Epifluoresence micrographs of late larval/young adult Q40 animals illustrating various numbers of aggregates in different animals. (Bar = 0.1 mm.) (F) Comparison of motility and aggregate number in adult Q40 animals (squares) and nontransgenic siblings (circles, no aggregates). Aggregate number is representative of number of muscle cells affected as the body-wall muscle cells had, on average, 1.3 ± 0.6 aggregates per cell (n = 212).
Fig 4.
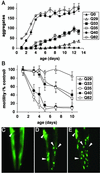
Influence of aging on polyQ aggregation and toxicity. (A) Accumulation of aggregates in Q82 (○), Q40 (•), Q35 (□), Q33 (▪), Q29 (▵), and Q0 (▴) during aging. Data are mean ± SEM. Twenty-four animals of each type are represented at day 1. Cohort sizes decreased as animals died during the experiment, but each data point represents at least five animals. (B) Motility index as a function of age for the same cohorts of animals described in A. Data are mean ± SD as a percentage of age-matched Q0 animals. (C_–_E) Epifluorescence micrographs of the head of an individual Q35 animal at 4 (C), 7 (D), and 10 (E) days of age, illustrating age-dependent accumulation of aggregates. Arrowheads indicate positions of the same aggregates on different days. In E, the animal is rotated slightly relative to its position in D.
Fig 5.
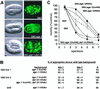
An extended lifespan mutation delays polyQ aggregate accumulation and onset of toxicity. (A) Differential interference contrast (Left) and epifluorescence (Right) micrographs showing embryos expressing Q82 in wild-type (Top), age-1(hx546) (Middle), or age-1(hx546);daf-16(RNAi) (Bottom) genetic backgrounds. (Bars = 5 μm.) (B) Aggregate accumulation in larval animals expressing Q40 or Q82 in the indicated genetic backgrounds relative to aggregate accumulation in wild-type background. Mean ± SEM. (C) Motility index for animals expressing Q40 or Q82 in the indicated genetic backgrounds. Data are mean ± SEM for 30 animals of each type. Motility of nontransgenic wild-type and age-1 animals was similar to that of wild-type (N2).
Similar articles
- Modeling polyglutamine pathogenesis in C. elegans.
Brignull HR, Morley JF, Garcia SM, Morimoto RI. Brignull HR, et al. Methods Enzymol. 2006;412:256-82. doi: 10.1016/S0076-6879(06)12016-9. Methods Enzymol. 2006. PMID: 17046663 - An apparent core/shell architecture of polyQ aggregates in the aging Caenorhabditis elegans neuron.
Fisher RS, Jimenez RM, Soto E, Kalev D, Elbaum-Garfinkle S. Fisher RS, et al. Protein Sci. 2021 Jul;30(7):1482-1486. doi: 10.1002/pro.4105. Epub 2021 May 22. Protein Sci. 2021. PMID: 33966305 Free PMC article. - Suppression of polyglutamine-induced toxicity in cell and animal models of Huntington's disease by ubiquilin.
Wang H, Lim PJ, Yin C, Rieckher M, Vogel BE, Monteiro MJ. Wang H, et al. Hum Mol Genet. 2006 Mar 15;15(6):1025-41. doi: 10.1093/hmg/ddl017. Epub 2006 Feb 6. Hum Mol Genet. 2006. PMID: 16461334 - Towards the treatment of polyglutamine diseases: the modulatory role of protein context.
Robertson AL, Bottomley SP. Robertson AL, et al. Curr Med Chem. 2010;17(27):3058-68. doi: 10.2174/092986710791959800. Curr Med Chem. 2010. PMID: 20629626 Review. - Genetic and pharmacological suppression of polyglutamine-dependent neuronal dysfunction in Caenorhabditis elegans.
Parker JA, Holbert S, Lambert E, Abderrahmane S, Néri C. Parker JA, et al. J Mol Neurosci. 2004;23(1-2):61-8. doi: 10.1385/JMN:23:1-2:061. J Mol Neurosci. 2004. PMID: 15126693 Review.
Cited by
- The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug.
Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. Johnson SM, et al. J Mol Biol. 2012 Aug 10;421(2-3):185-203. doi: 10.1016/j.jmb.2011.12.060. Epub 2012 Jan 5. J Mol Biol. 2012. PMID: 22244854 Free PMC article. Review. - Somatic expression of unc-54 and vha-6 mRNAs declines but not pan-neuronal rgef-1 and unc-119 expression in aging Caenorhabditis elegans.
Adamla F, Ignatova Z. Adamla F, et al. Sci Rep. 2015 Jun 2;5:10692. doi: 10.1038/srep10692. Sci Rep. 2015. PMID: 26031360 Free PMC article. - FOXOs: signalling integrators for homeostasis maintenance.
Eijkelenboom A, Burgering BM. Eijkelenboom A, et al. Nat Rev Mol Cell Biol. 2013 Feb;14(2):83-97. doi: 10.1038/nrm3507. Epub 2013 Jan 17. Nat Rev Mol Cell Biol. 2013. PMID: 23325358 Review. - PolyQ disease: misfiring of a developmental cell death program?
Blum ES, Schwendeman AR, Shaham S. Blum ES, et al. Trends Cell Biol. 2013 Apr;23(4):168-74. doi: 10.1016/j.tcb.2012.11.003. Epub 2012 Dec 8. Trends Cell Biol. 2013. PMID: 23228508 Free PMC article. Review. - O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases.
Wang P, Lazarus BD, Forsythe ME, Love DC, Krause MW, Hanover JA. Wang P, et al. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17669-74. doi: 10.1073/pnas.1205748109. Epub 2012 Sep 17. Proc Natl Acad Sci U S A. 2012. PMID: 22988095 Free PMC article.
References
- Kopito R. R. & Ron, D. (2000) Nat. Cell Biol. 2, E207-E209. - PubMed
- Horwich A. L. & Weissman, J. S. (1997) Cell 89, 499-510. - PubMed
- Perutz M. F. (1999) Trends Biochem. Sci. 24, 58-63. - PubMed
- Orr H. T. (2001) Genes Dev. 15, 925-932. - PubMed
- Mangiarini L., Sathasivam, K., Seller, M., Cozens, B., Harper, A., Hetherington, C., Lawton, M., Trottier, Y., Lehrach, H., Davies, S. W. & Bates, G. P. (1996) Cell 87, 493-506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM038109/GM/NIGMS NIH HHS/United States
- GM38109/GM/NIGMS NIH HHS/United States
- T32 GM08061/GM/NIGMS NIH HHS/United States
- T32 GM008061/GM/NIGMS NIH HHS/United States
- R01 GM038109/GM/NIGMS NIH HHS/United States
- T32 GM008152/GM/NIGMS NIH HHS/United States
- GM08152/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical